Here we have shared class 12 Chemistry Amines Notes. The Amines notes is a best resources for students who are preparing for their board exam because it compile the entire lesson into short and includes every important topics.
With the help of Amines notes students can understand the chapter in a better way. Notes are prepared by very experience teachers in an organised way so students can rely on this notes for their exam preparation.
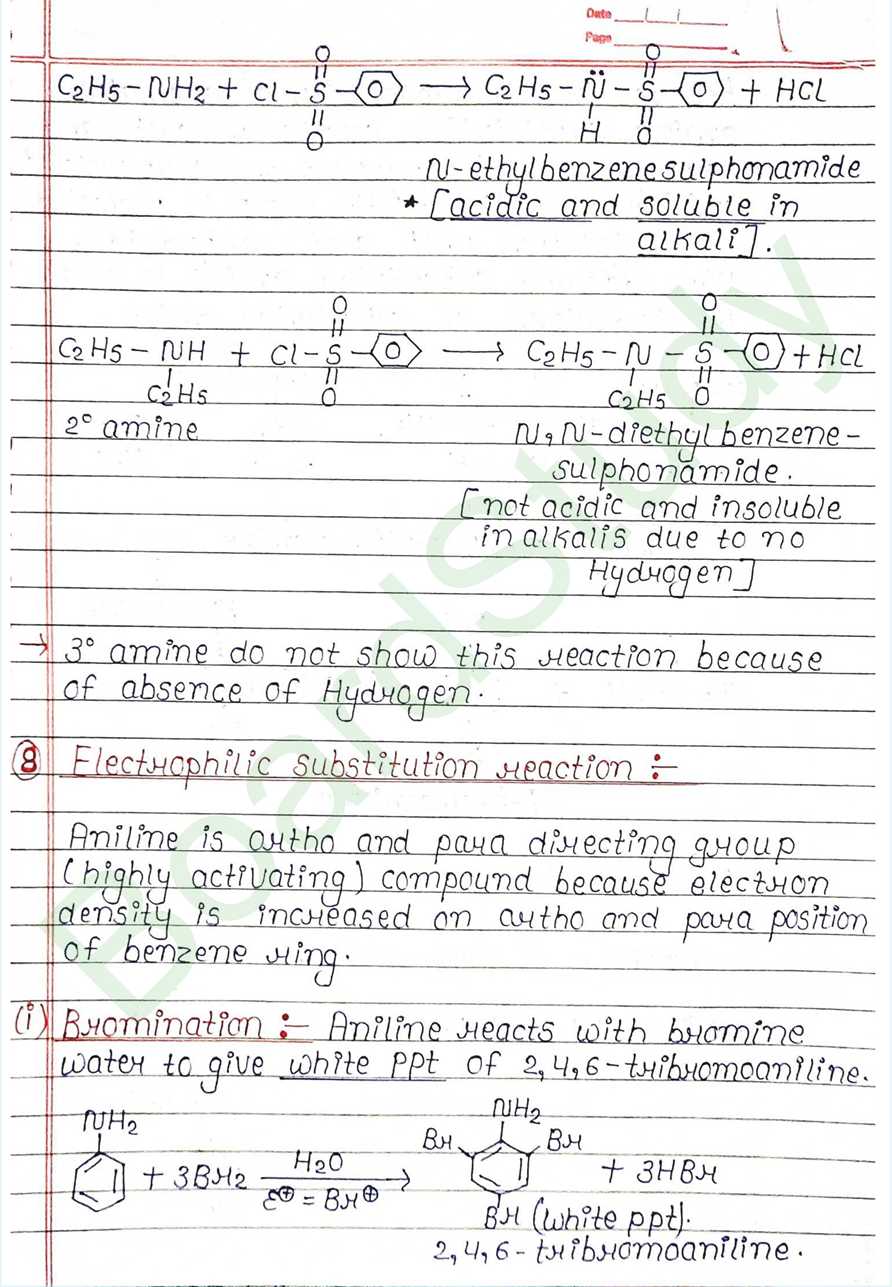
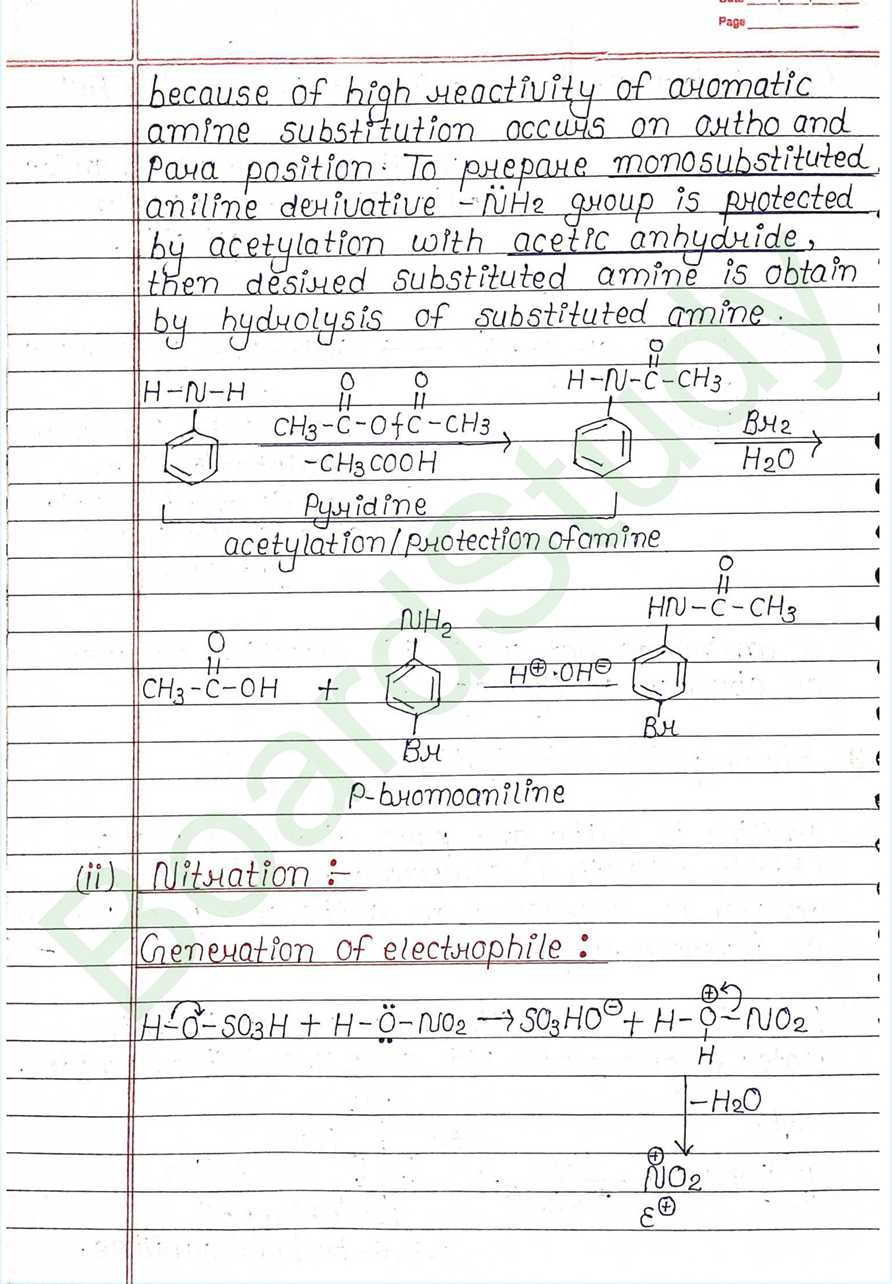
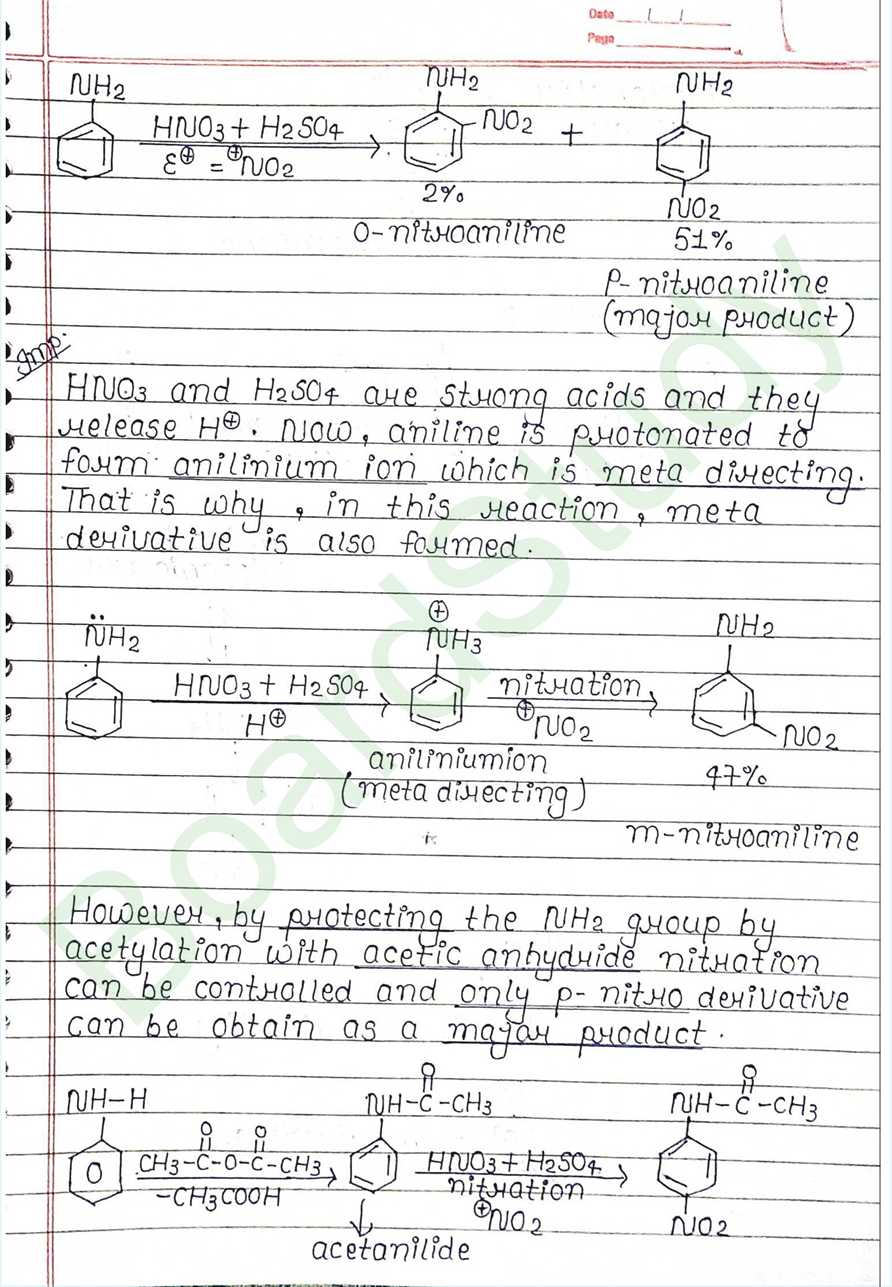
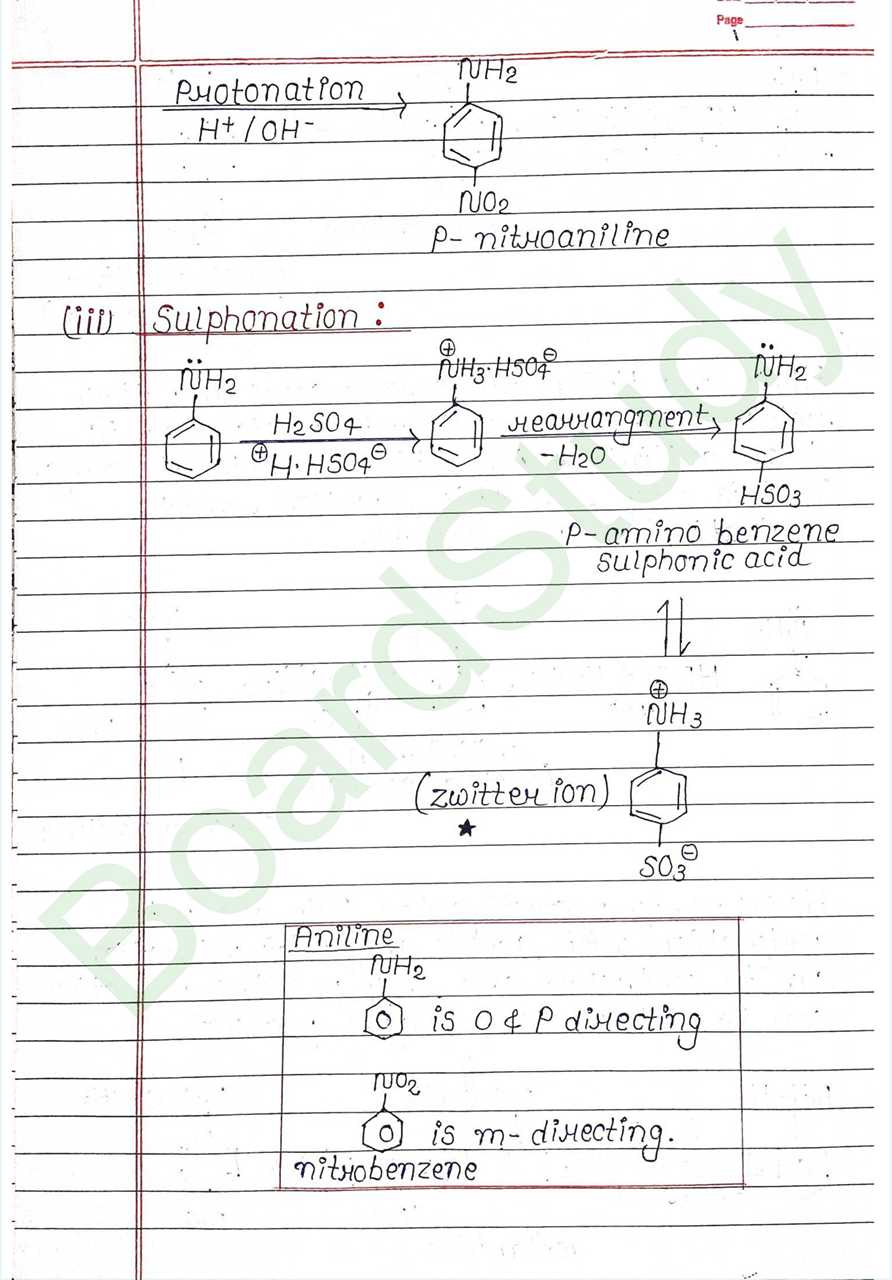
Class 12 Chemistry Amines Handwritten Notes






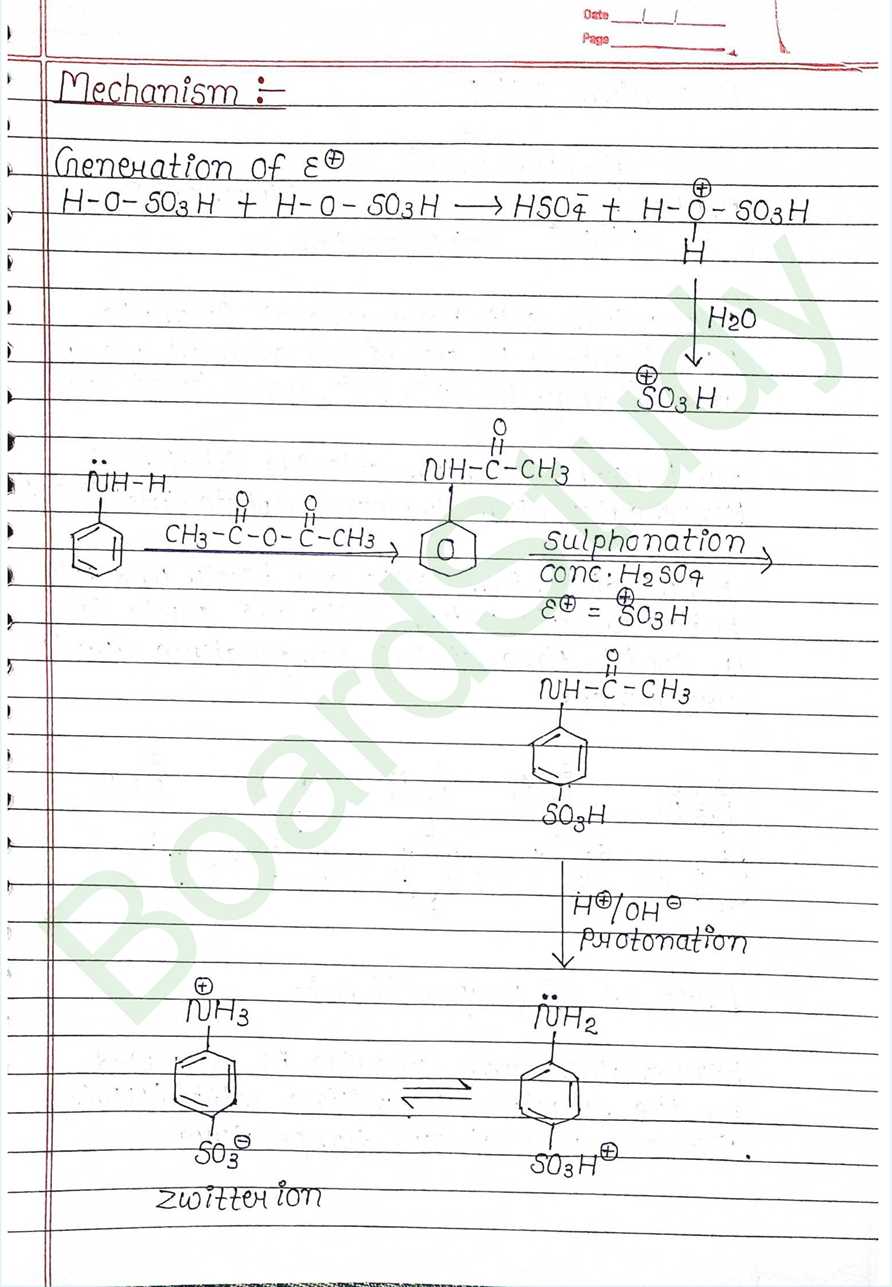
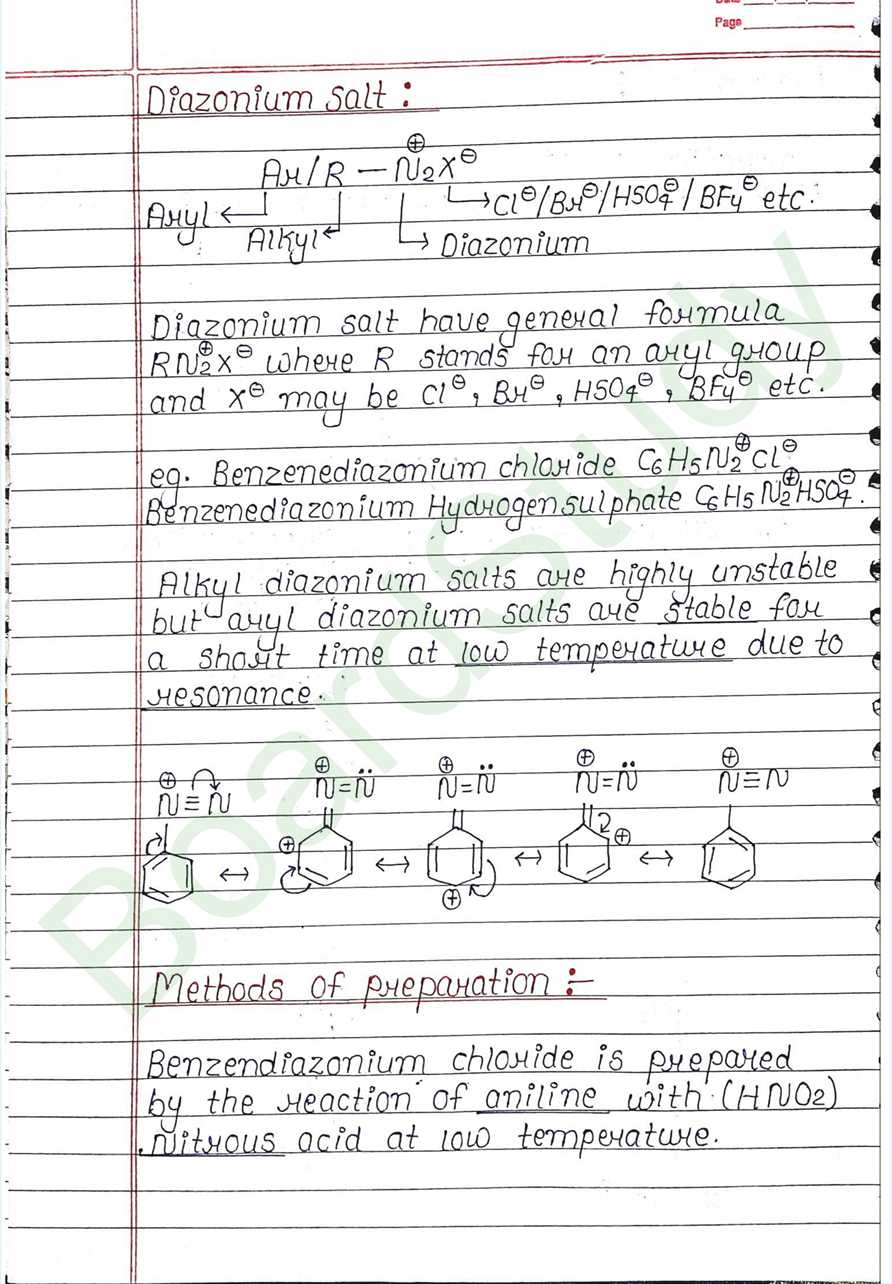

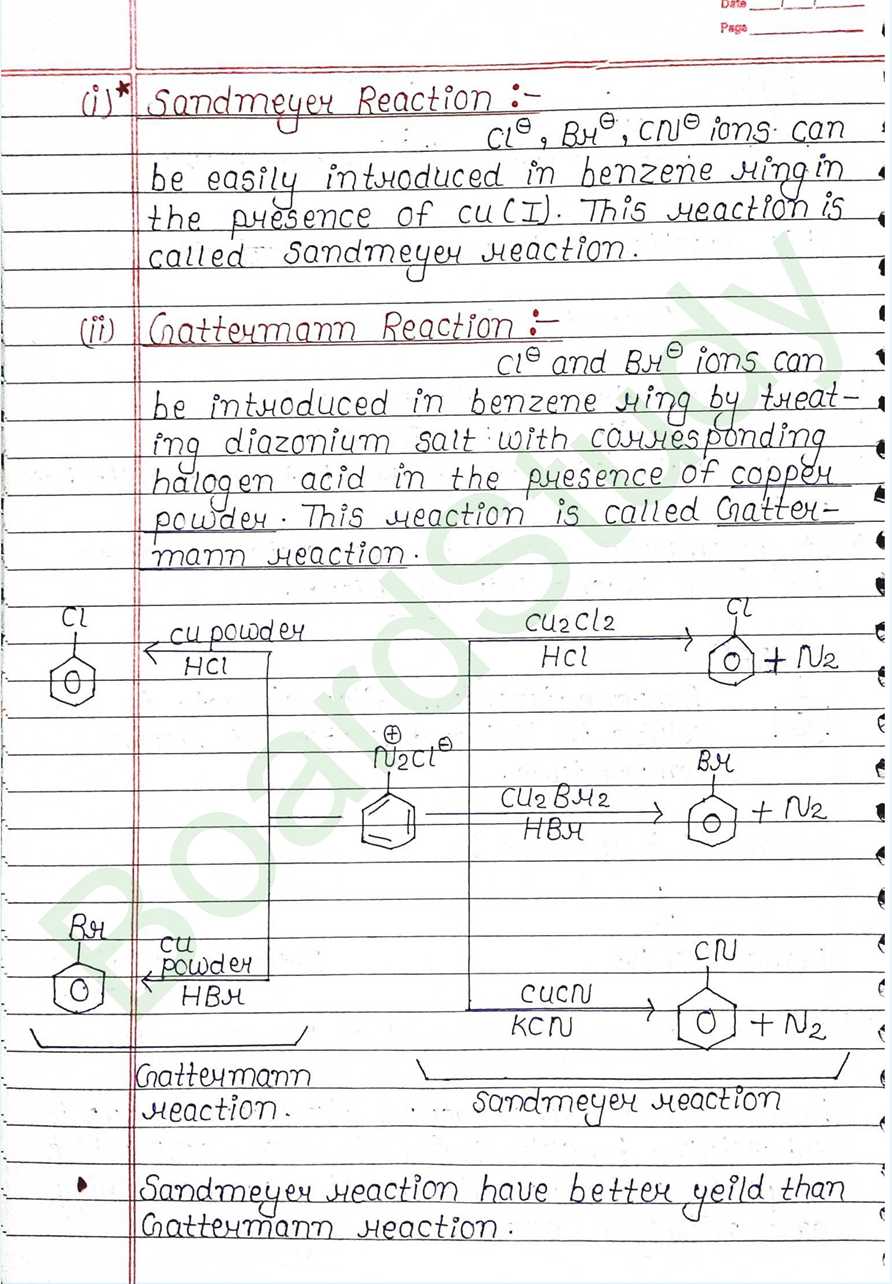
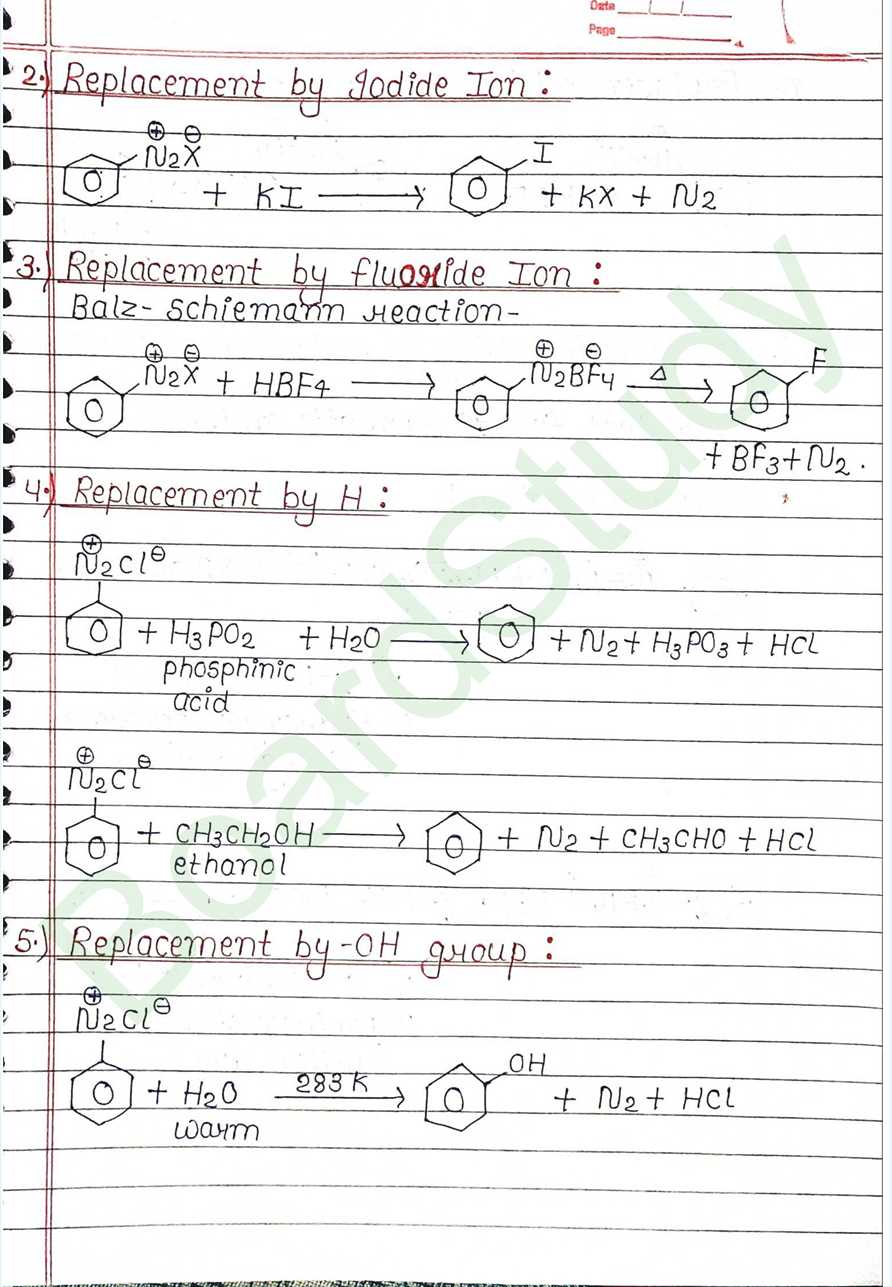
Next Chapter: Biomolecules
Previous Chapter: Aldehydes, Ketones, and Carboxylic Acids
Other Subjects:
Class 12 Biology Notes
Class 12 Physics Notes
Students can access this notes anytime on our website for free of cost. If you found notes helpful, you can also help your friends by sharing with them.
Key Points: Amines Notes PDF
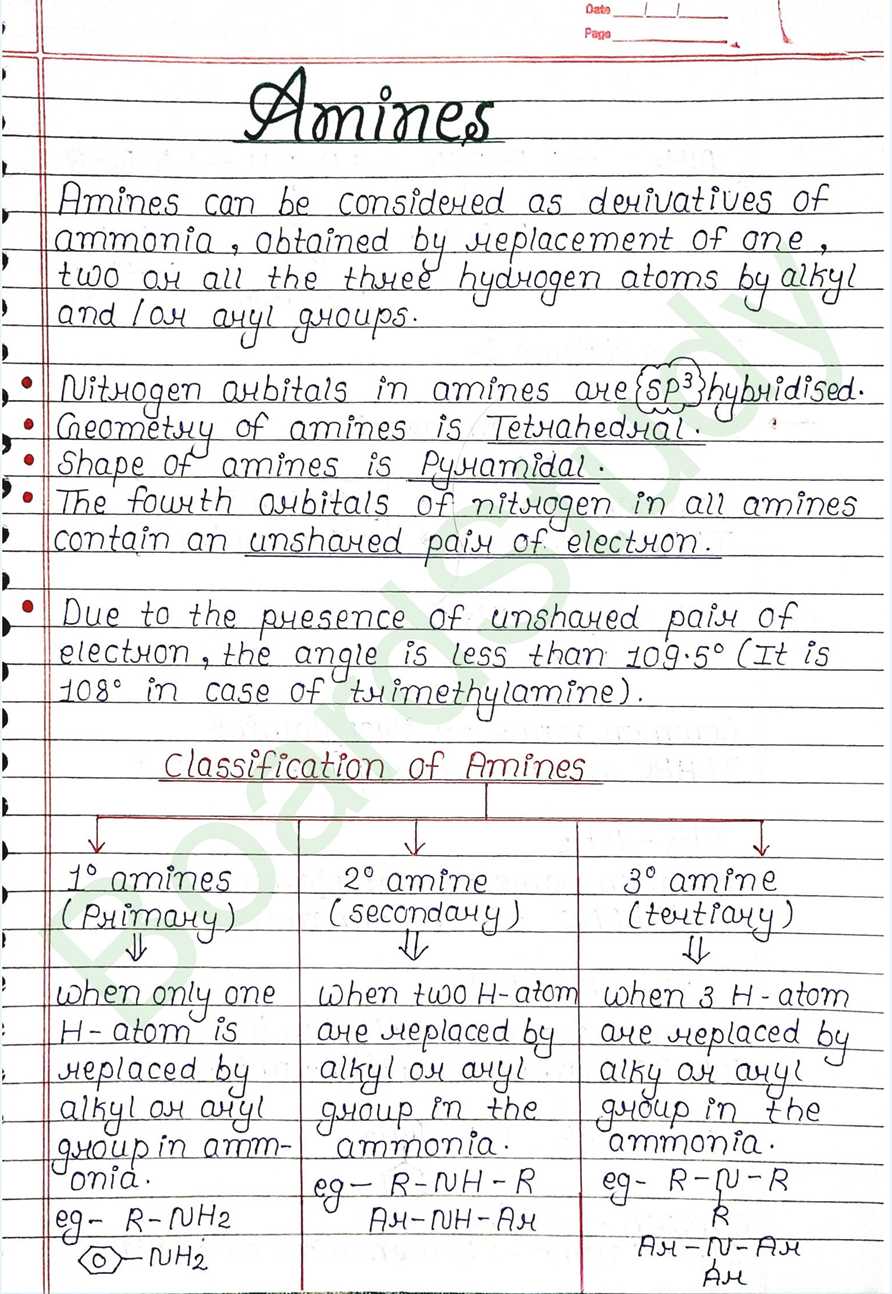
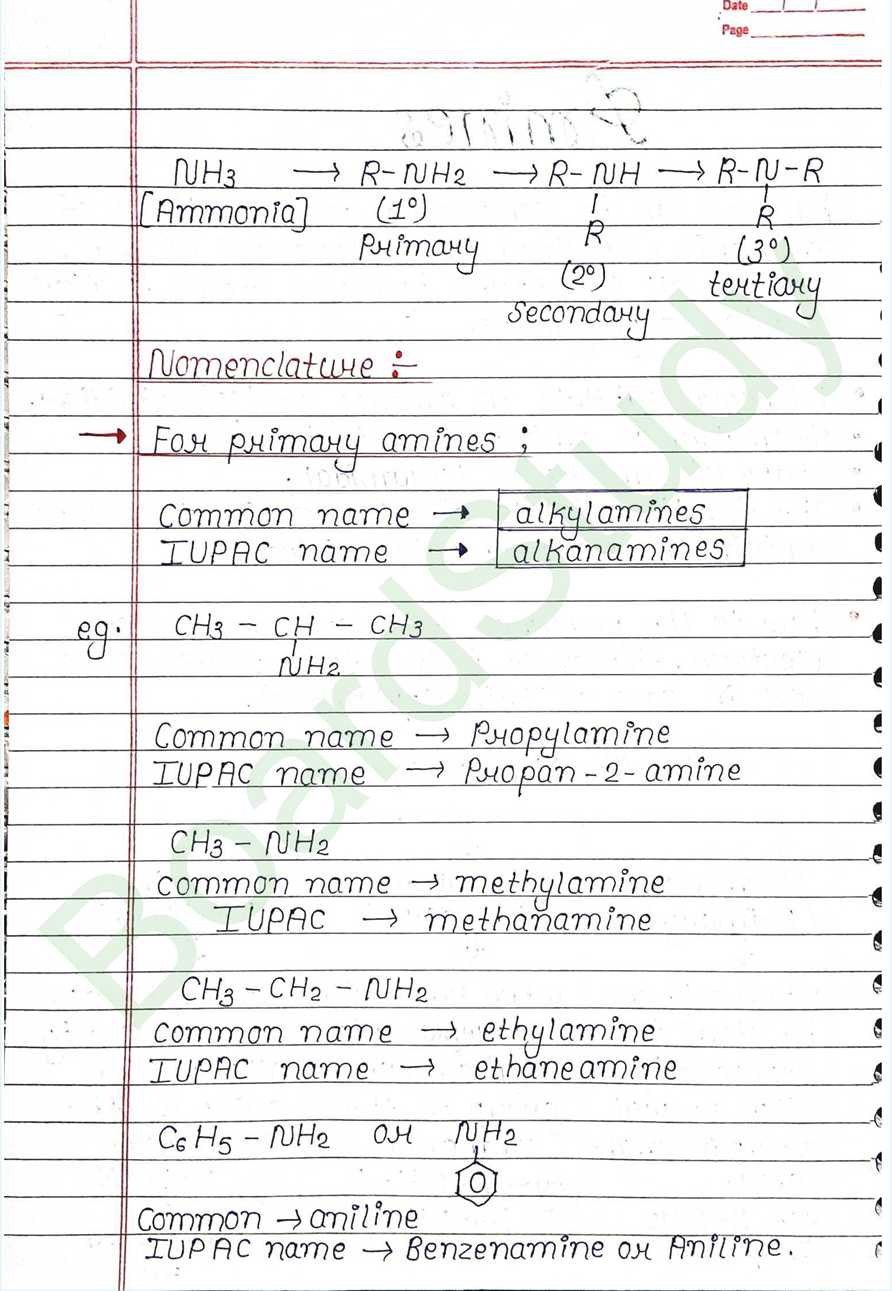
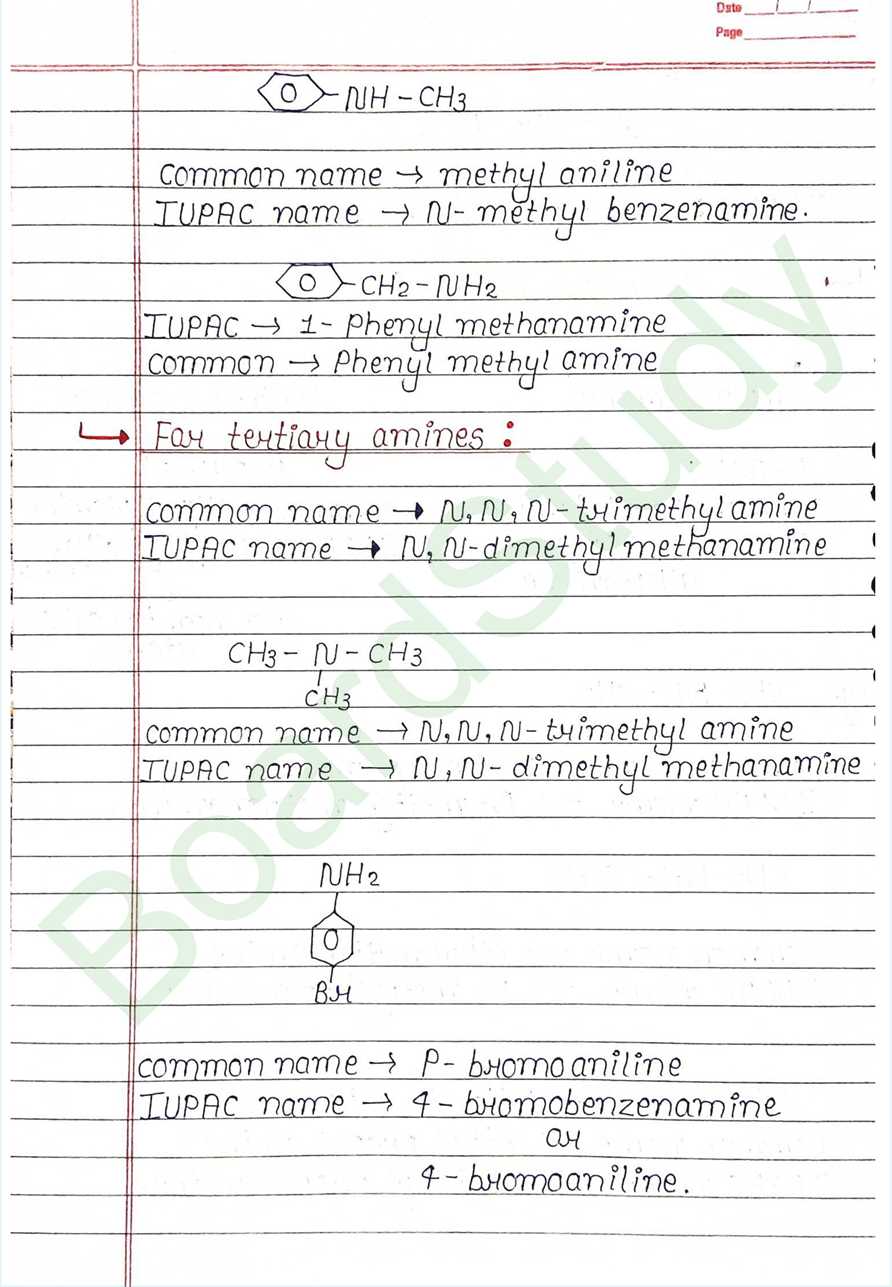
Amines can be considered as derivatives of ammonia, obtained by replacement of one, two or all the three hydrogen atoms by alkyl and or aryl groups.
- Nitrogen orbitals in amines are sp3 hybridised.
- Geometry of amines is Tetrahedral.
- Shape of amines is Pyramidal.
- The fourth orbitals of nitrogen in all amines contain an unshared pair of electron.
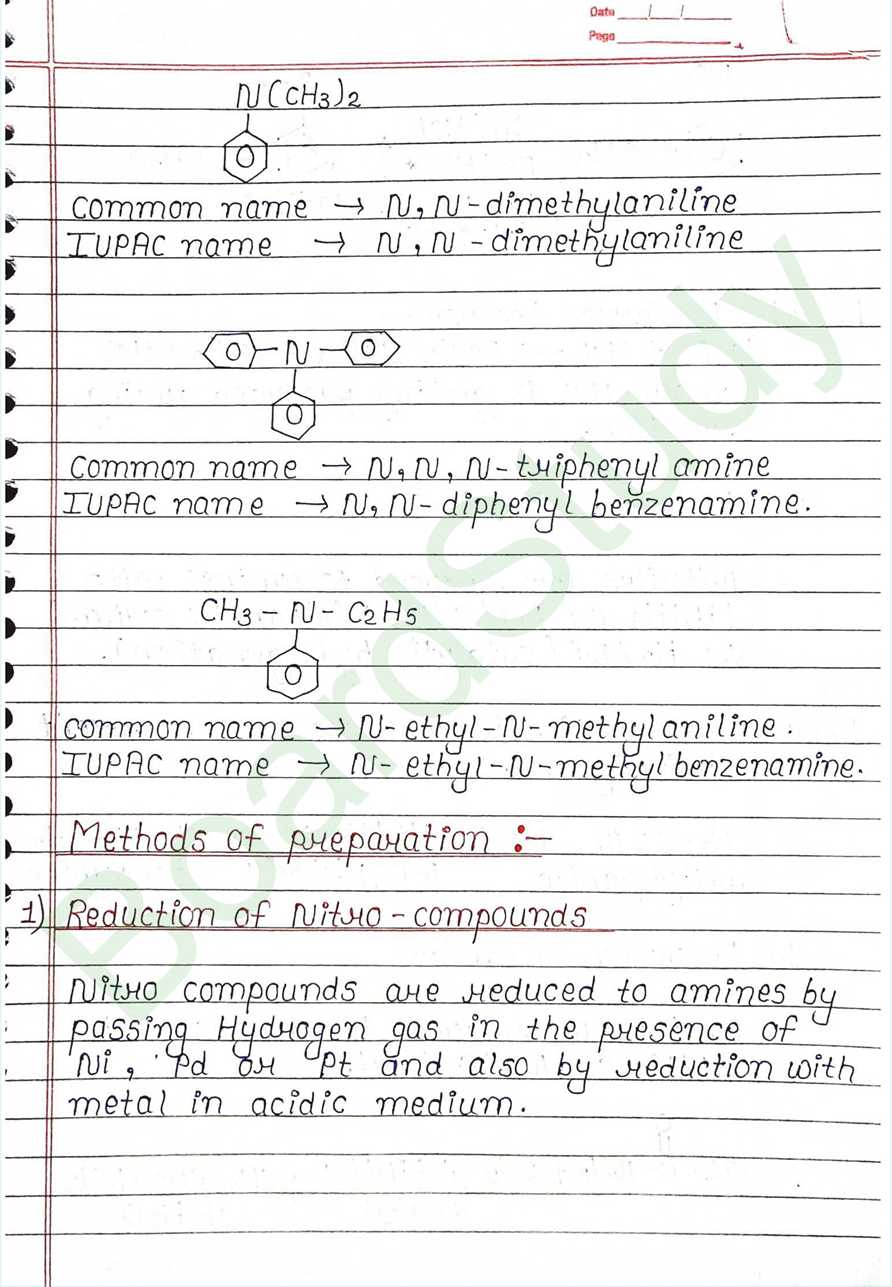
Methods of preparation
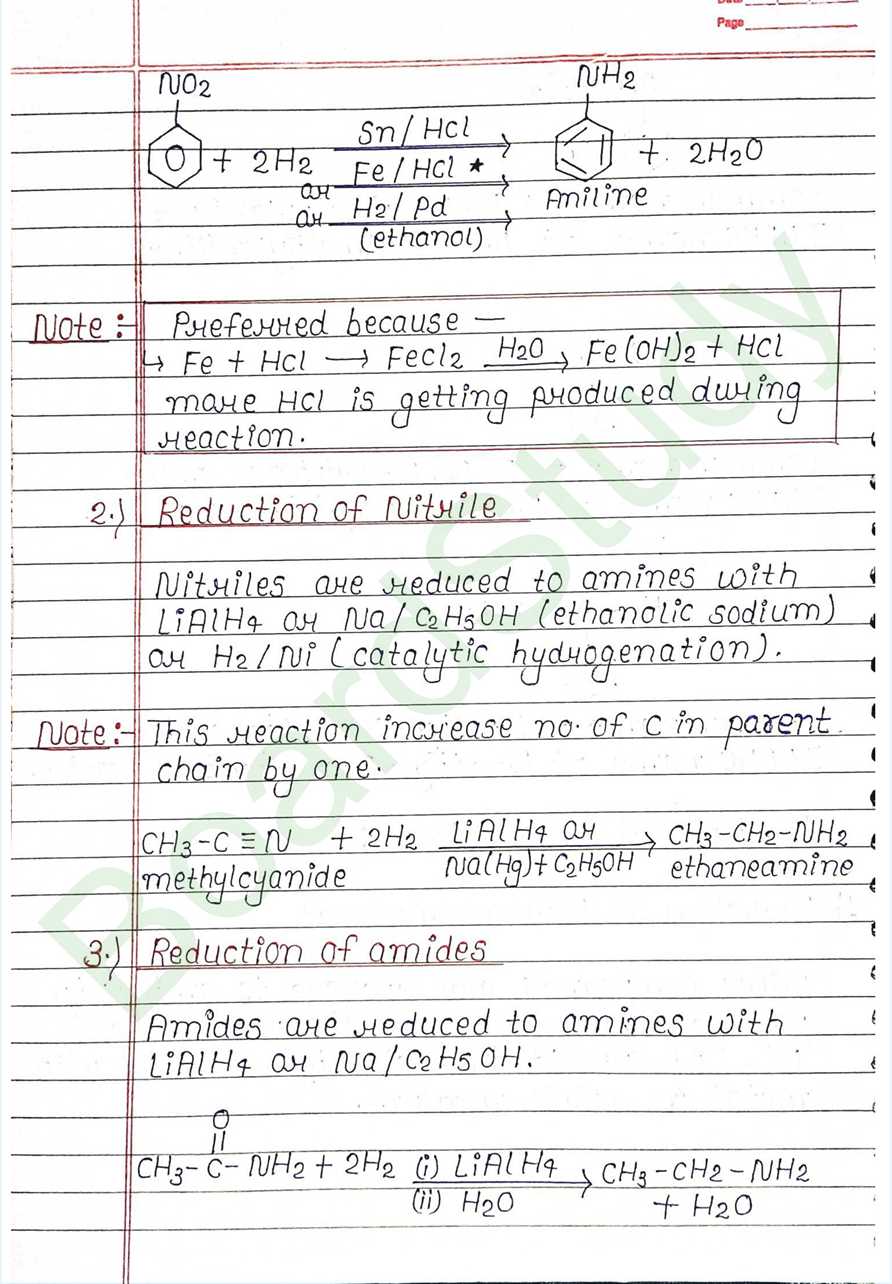
- Reduction of Nitro-compounds: Nitro compounds are reduced to amines by passing Hydrogen gas in the presence of Ni, Pd or pt and also by reduction with metal in acidic medium.
- Reduction of Nitrile: Nitriles are reduced to amines with LiAlH4, Na/C2H5OH (ethanolic sodium), or H2 / Ni (catalytic hydrogenation).
- Reduction of amides: Amides are reduced to amines with LiAlH_4 or Na/C_2H_5OH
- Ammonolysis (Hoffmann’s Ammonolysis): When alkyl Halide (R-X) reacts with alcoholic solution of ammonia, halogen atom of alkyl halide is replaced by -NH2 group of ammonia. This process of cleavage of R-X by ammonia is known as ammonolysis.
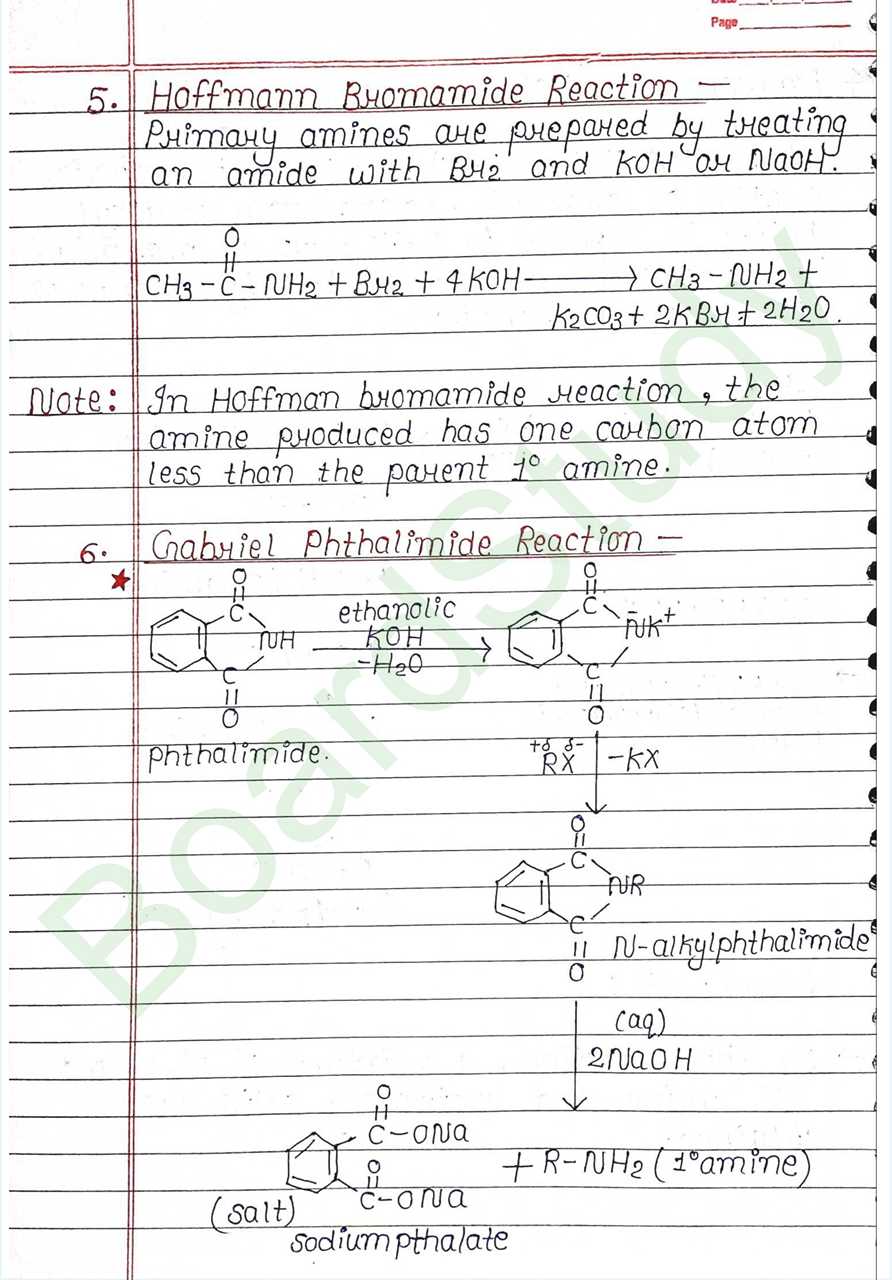
- Hoffmann Bromamide Reaction: Primary amines are prepared by treating an amide with Br2 and KOH or NaOH.
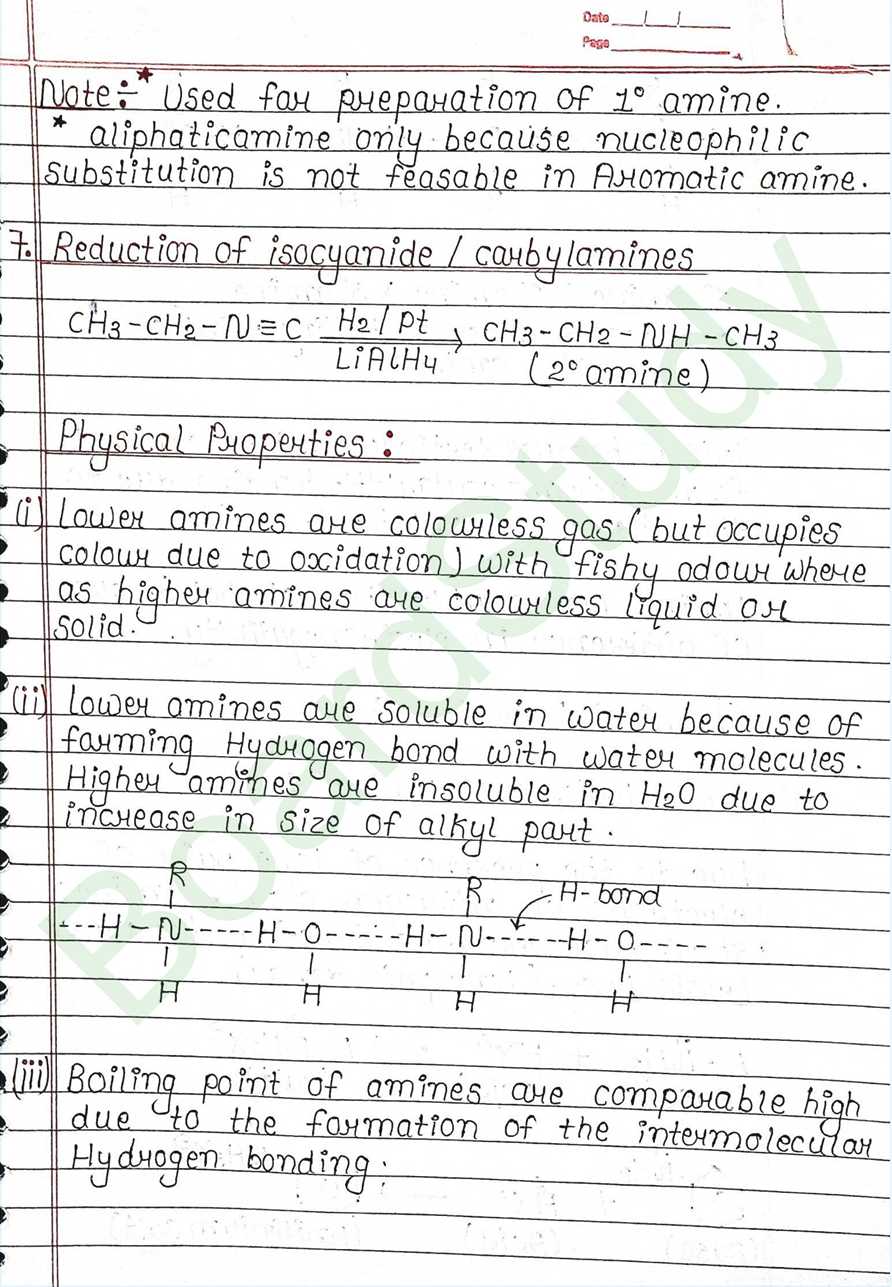
Physical Properties:
- Lower amines are colourless gas (but occupies colour due to oxidation) with fishy odour where as higher amines are colourless liquid or Solid.
- Lower amines are soluble in water because of forming Hydrogen bond with water molecules.
- Higher amines are insoluble in H₂O due to increase in size of alkyl part.
- Boiling point of amines are comparable high due to the formation of the intermolecular Hydrogen bonding:
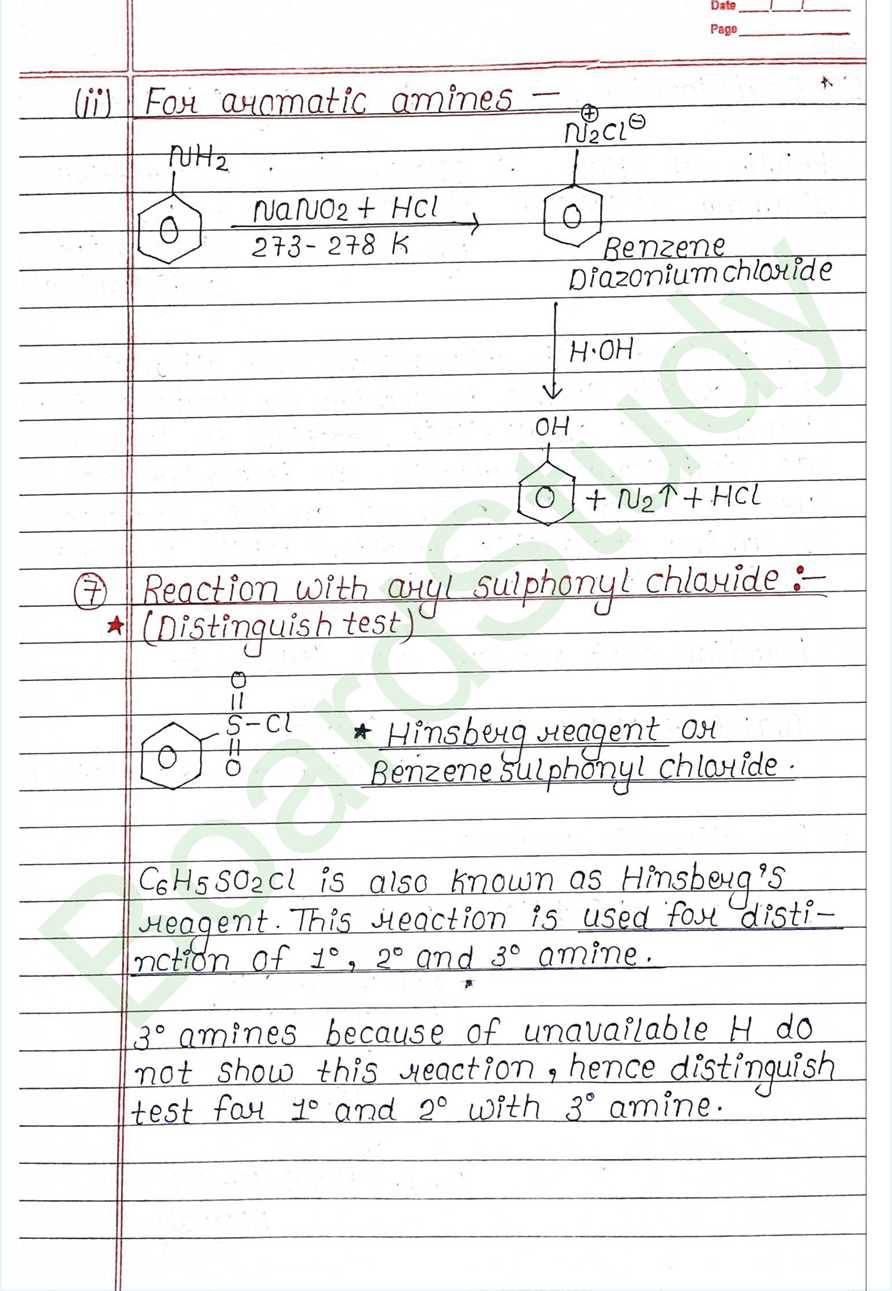
Chemical Properties :
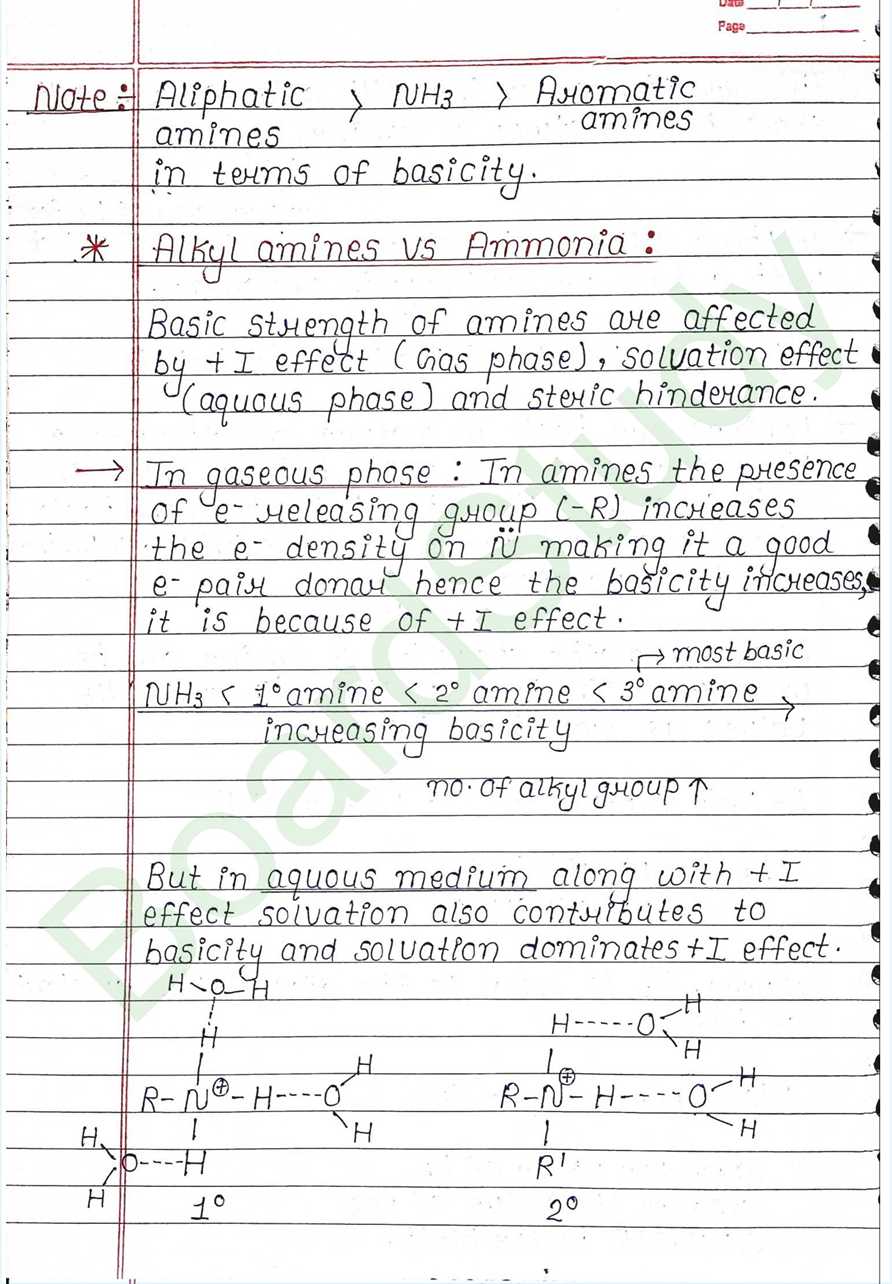
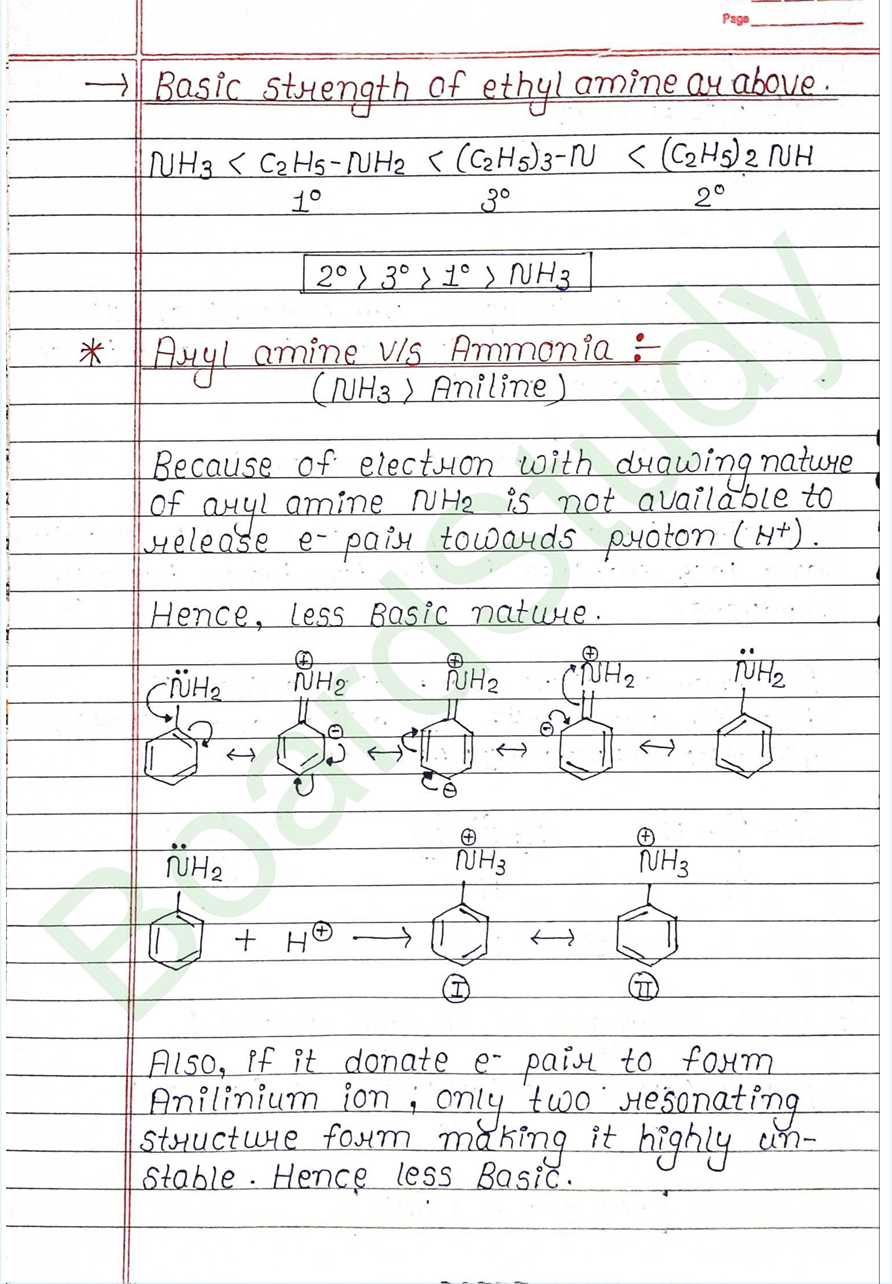
- Basic Nature of Amines
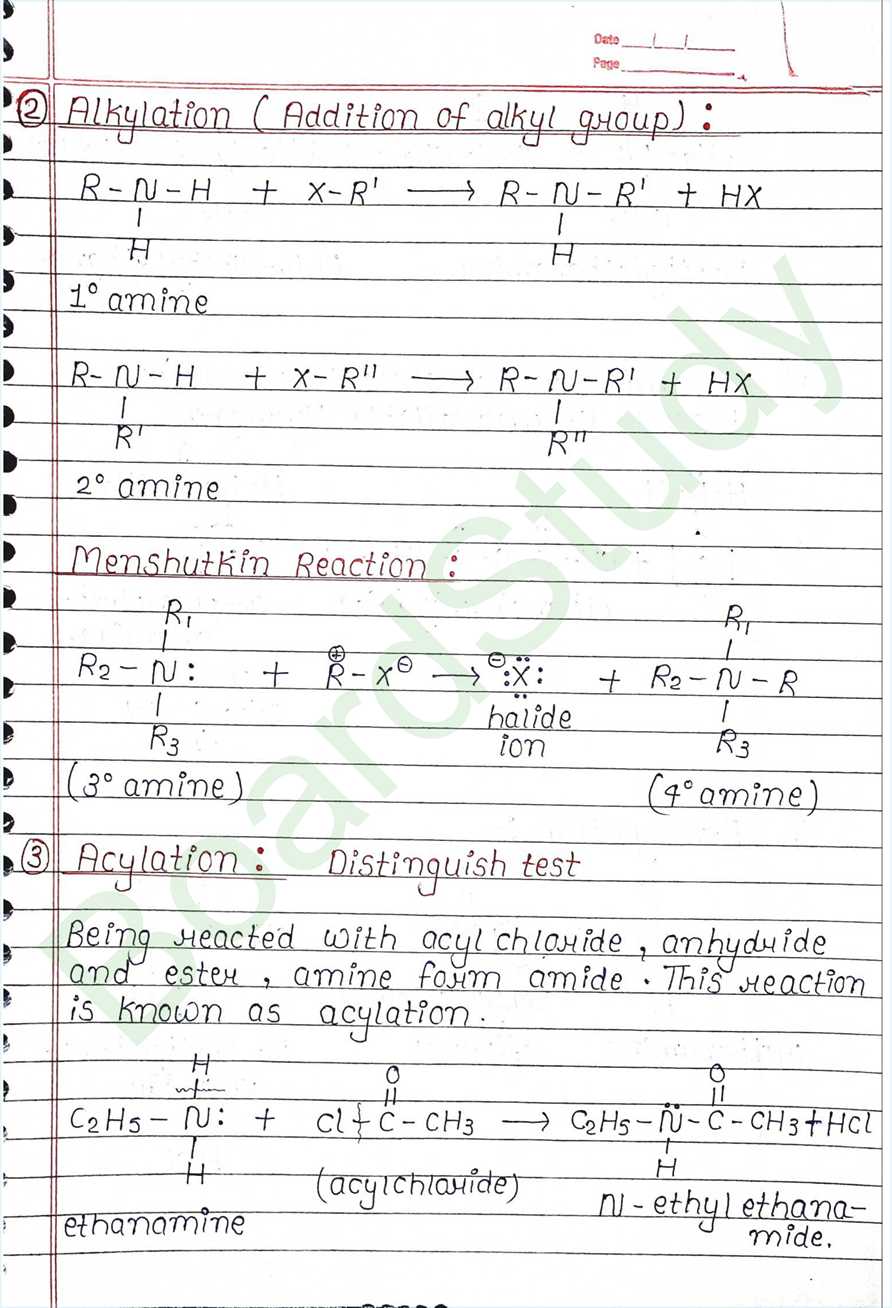
- Alkylation ( Addition of alkyl group)
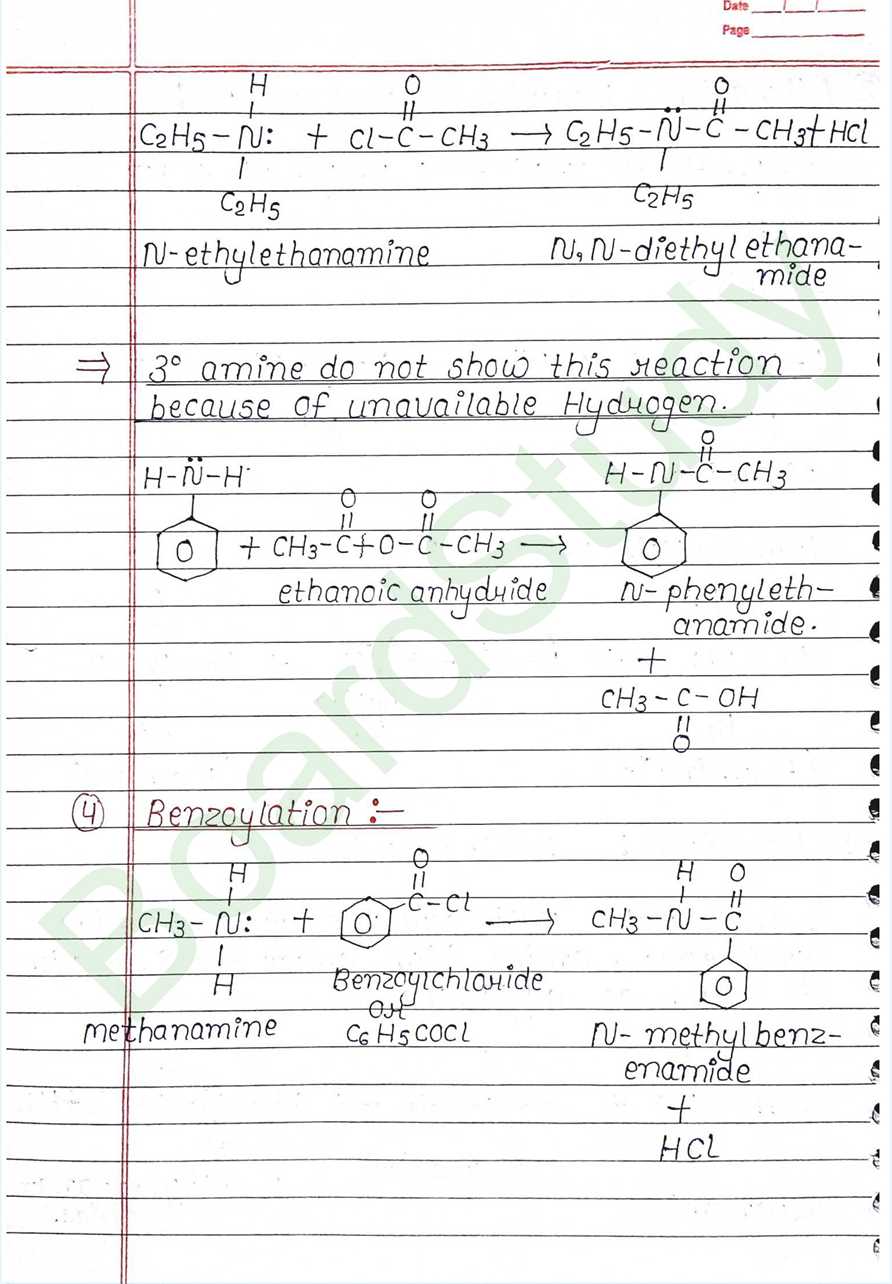
- Acylation: Distinguish test
- Benzoylation
- Carbylamine reaction
Features of Notes
- Students can use Amines notes for last minute revision.
- In the last few days of exam students feel very stress due to pressure of exam. Notes will be very helpful for managing the stress in the last days of exam.
- All notes are totally free of cost and students can access notes anytime on our for totally free of cost.
- Amines Notes PDF are created very carefully so you can rely on this notes.
Summary
| Chapter | Amines |
| Chapter Number | 9 |
| Subject | Chemistry |
| Class | 12 |
| Medium | English |
FAQ
What is Amines ?
Amines can be considered as derivatives of ammonia, obtained by replacement of one, two or all the three hydrogen atoms by alkyl and or aryl groups.
What is Hoffmann Bromamide Reaction ?
Primary amines are prepared by treating an amide with Br2 and KOH or NaOH.
Are these notes sufficient for board exam?
Amines handwritten notes are created by topper’s and expert teacher keeping board exam in mind so you can score maximum in board exam.
Are Haloalkanes and Amines notes according to NCERT latest syllabus?
Yes notes are created according to the NCERT latest syllabus.
How can i download Amines Notes PDF?
For downloading Amines Notes PDF click on Download PDF button.
