Physics is a very important subject for students. It may be hard for some students. For helping students here we have shared NCERT Class 12 Physics Chapter 12 Atoms Handwritten Notes.
The Atoms notes is a best resource for students who are preparing for their board exam because it compile the entire lesson in short and include every important topic.
NCERT Class 12 Physics Chapter 12 Atoms Notes







Next Chapter: Nuclei
Previous Chapter: Dual Nature of Radiation and Matter
Other Subjects:
Class 12 Biology Notes
Class 12 Chemistry Notes
Students who are studying physics in class 12 should definitely use this note and you can access this notes anytime on our website for free of cost. If you found notes helpful, you can also help your friends by sharing with them
Atoms Notes PDF
We have also shared the PDF of Atoms Notes so students don’t feel any difficulty in reading the notes. You can ask your doubts in the comment section. We will be happy to help you.
Key Points: Atoms Notes PDF
Atom : A atom is the smallest particle which can take part in all physical and chemical reactions.
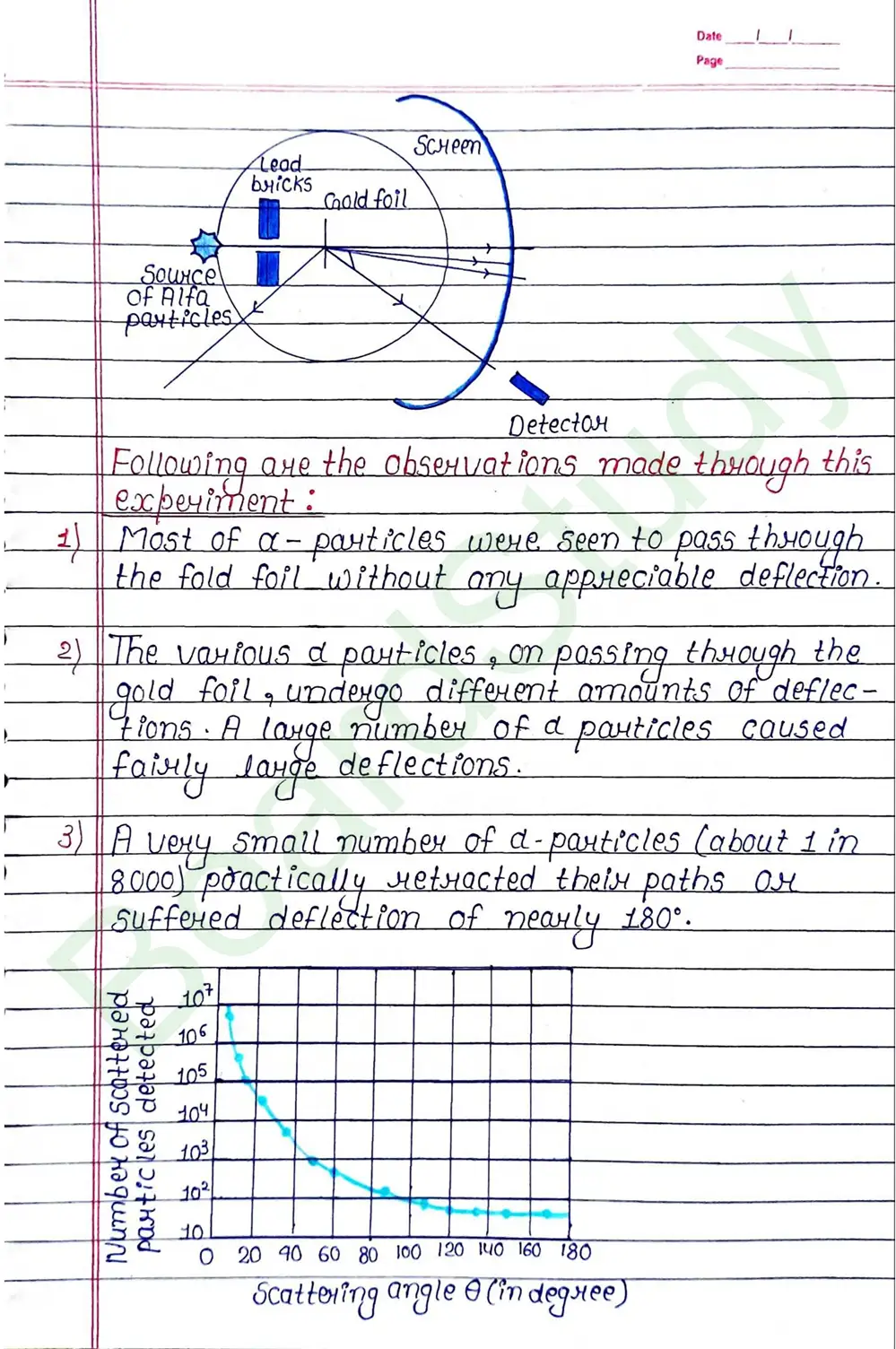
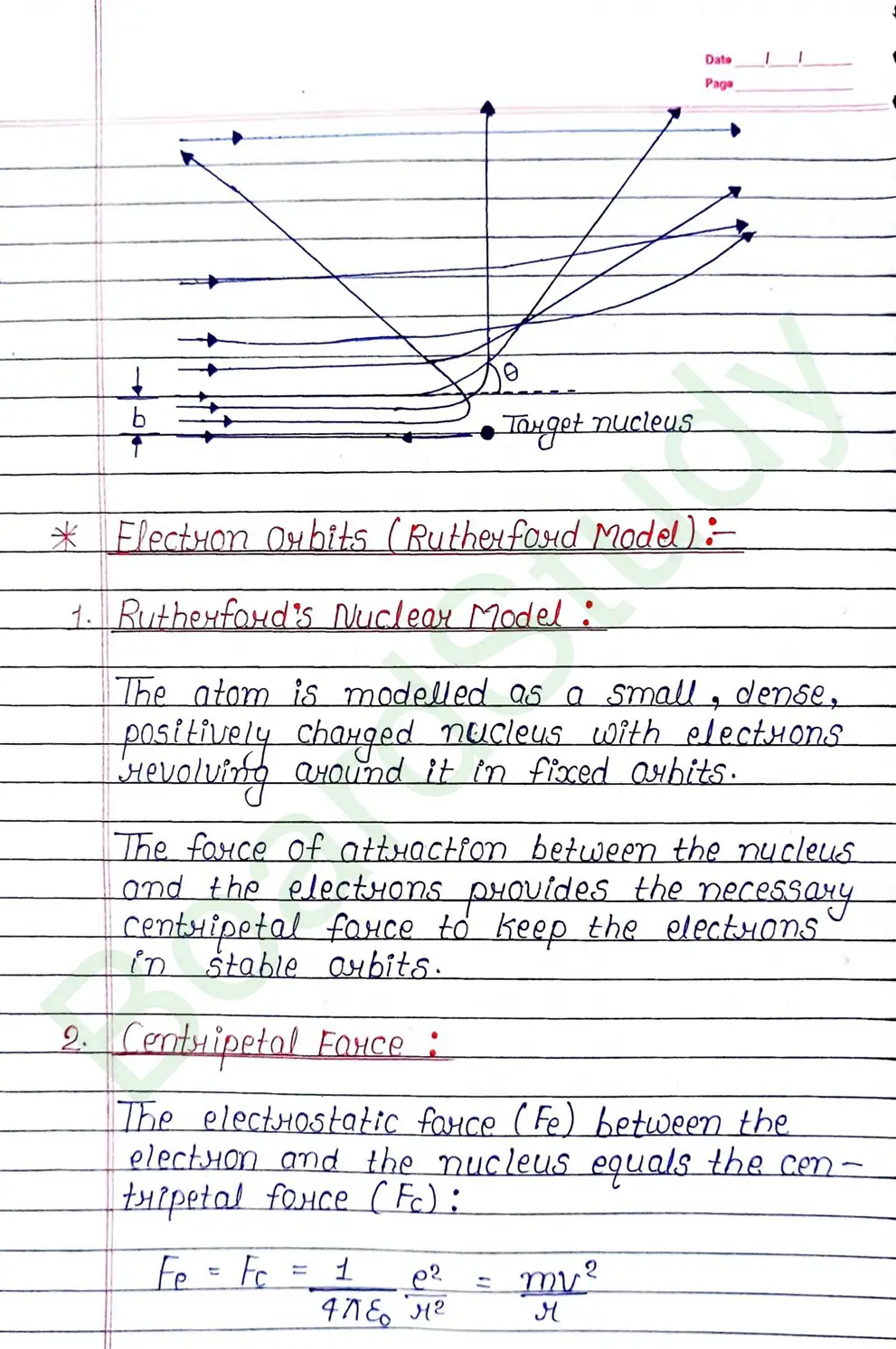
- Rutherford’s Atom Model : On the basis of the results of a scattering experiment the following picture of an atom:
- Atoms can be regarded as spheres of diameters
m but whole of the positive charge and almost the entire mass of these atoms are concentrated in small central cares called nuclei having diameters of about m.
- The nucleus is neighbored by electrons. In other wards, the electrons are distri- buted over the remaining part of the atom leaving plenty of empty space in the atom.
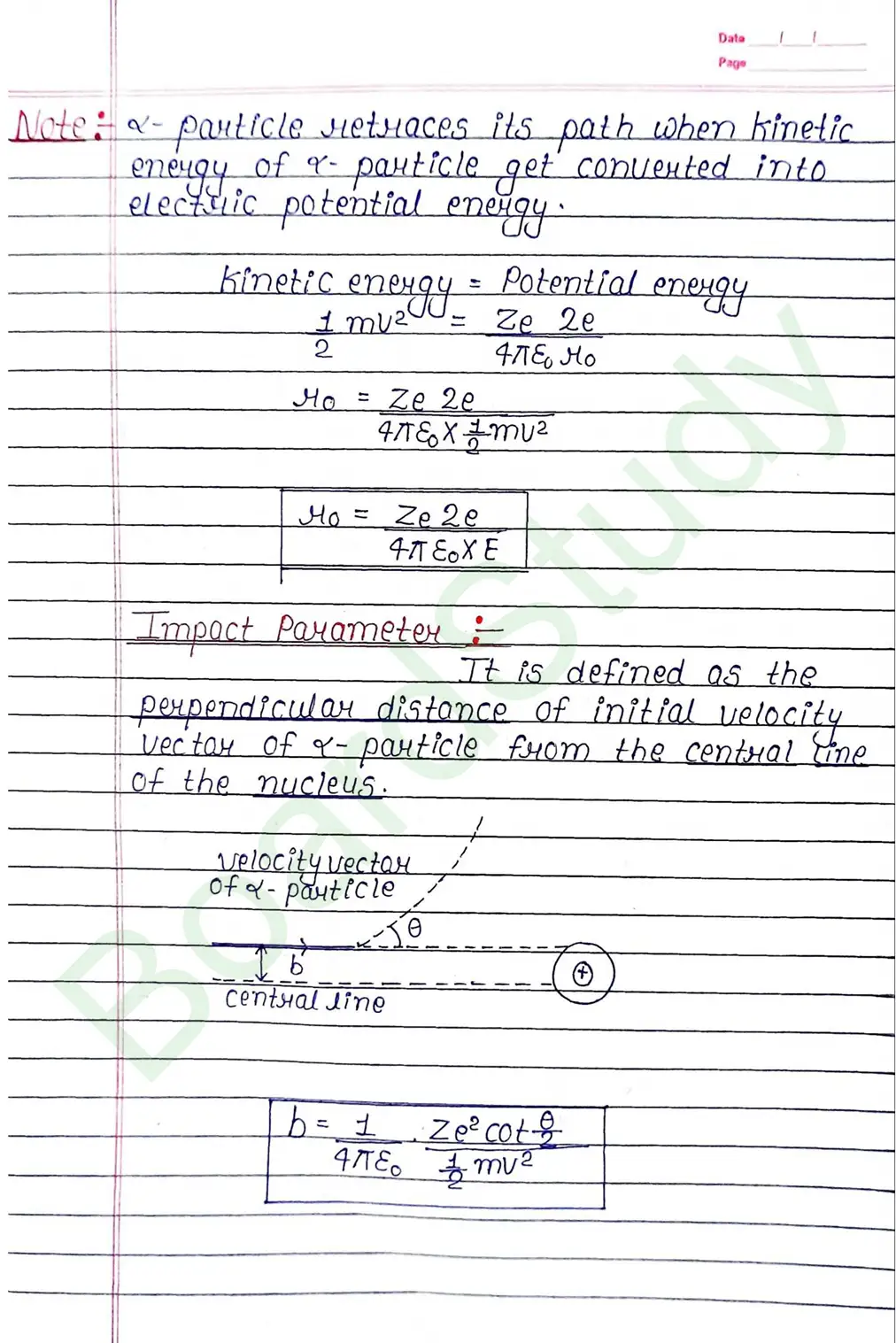
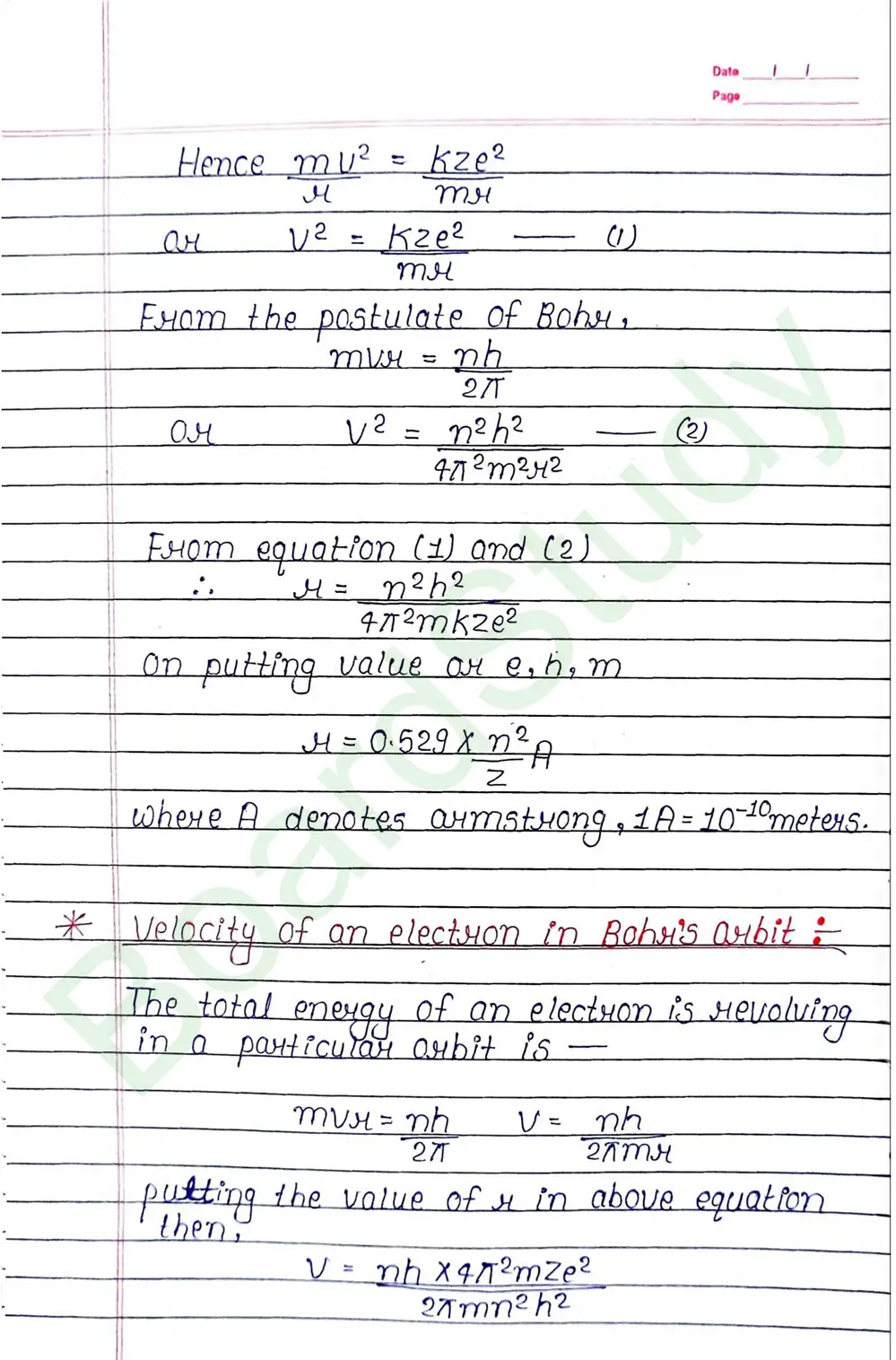
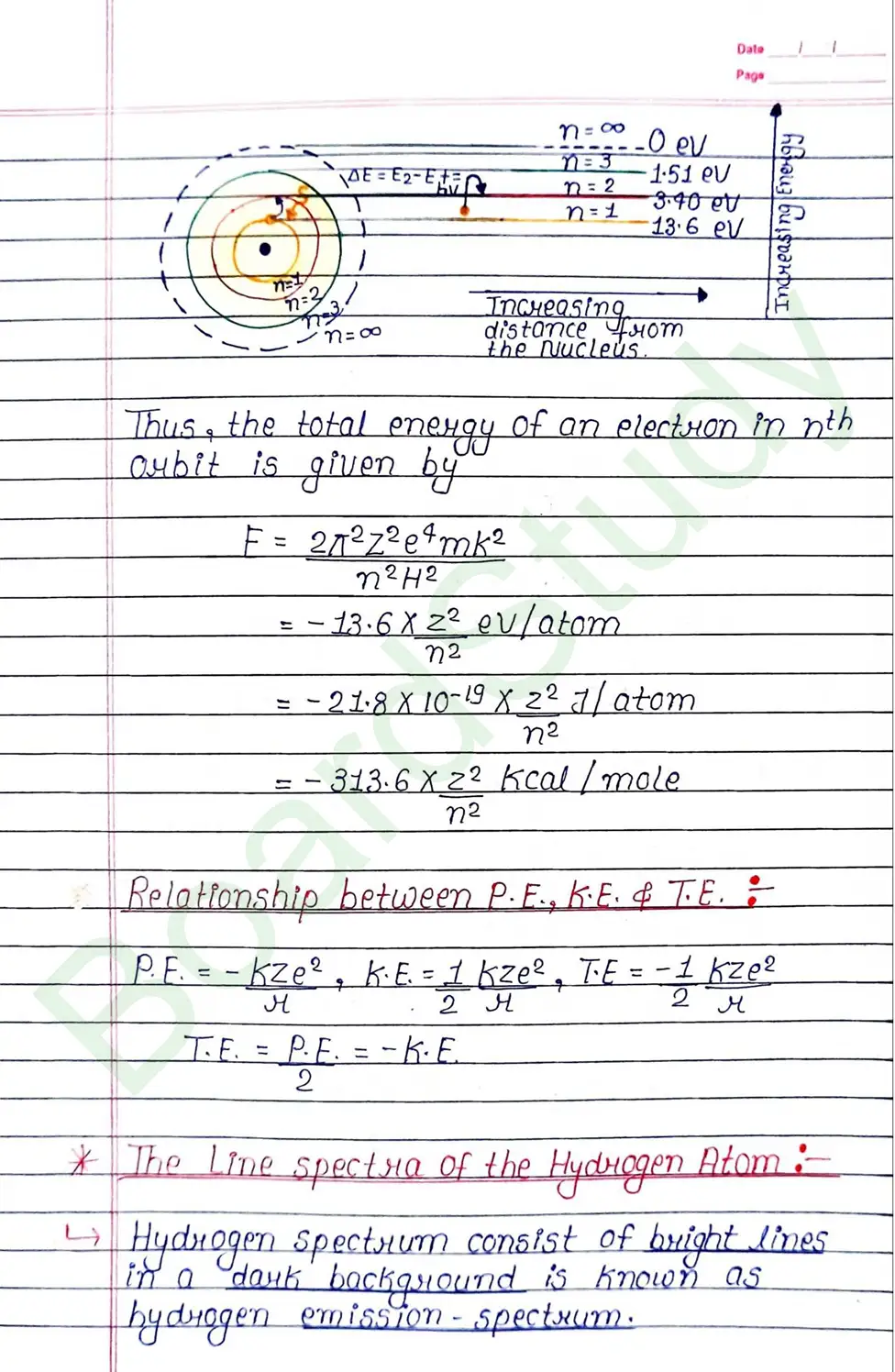
Bohr Model of Hydrogen atom:- Bohr combined classical and early quantum concepts and gave a theory in the form of
- According to Bohr, every atom consist of a central care called nucleus, in which entire positive charge and almost entire mass of atom is concentrated A suitable number of electron revolve around the nucleus in circular arbit.
- According to Bohr, e con revolve in certain non-radiating arbit called stationary arbit. for which the total angular momentum of revolving electron is integral multiple of h/
2𝜋.
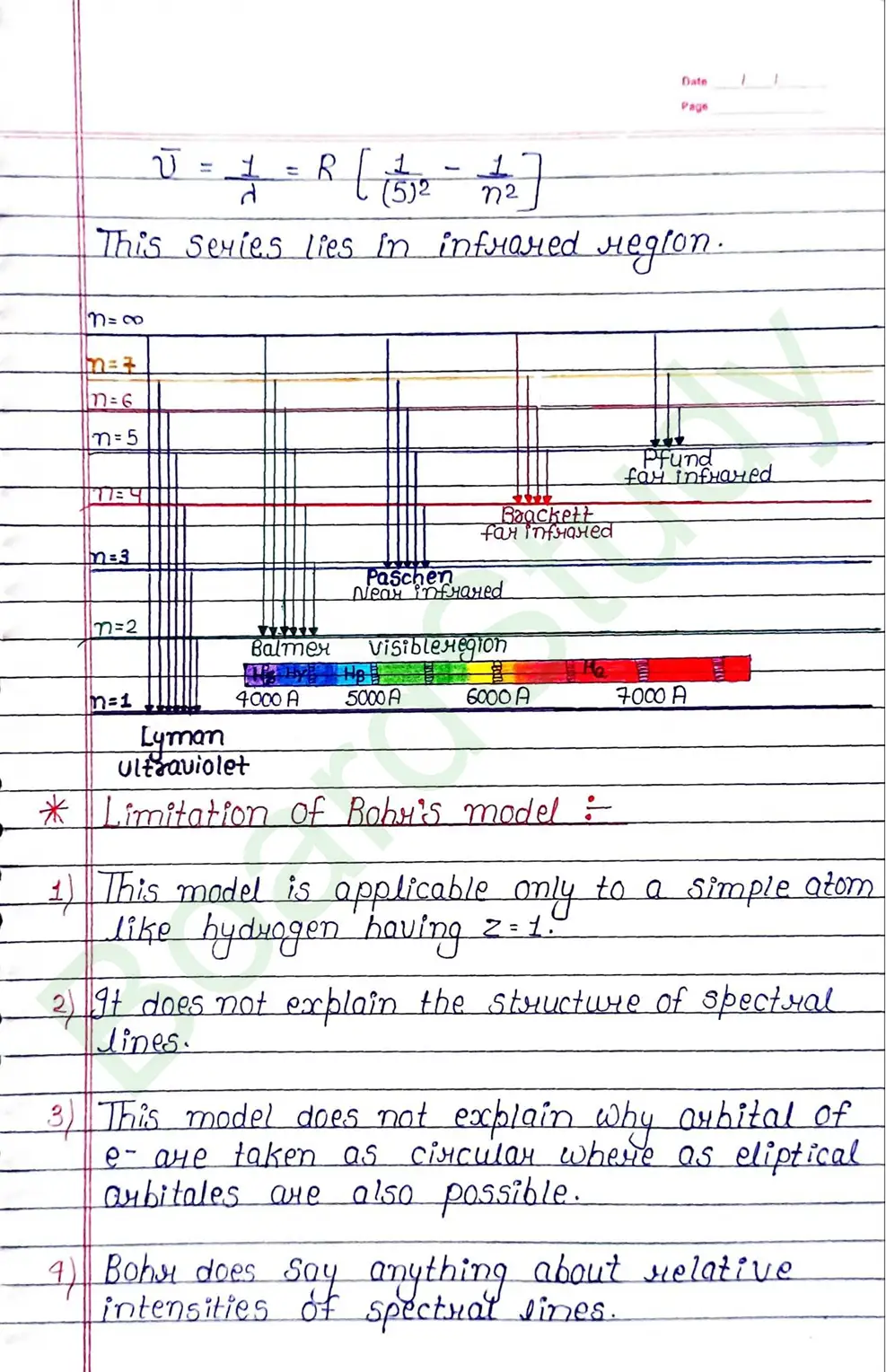
Limitation of Bohr’s model:
- This model is applicable only to a simple atom like hydrogen having Z=1
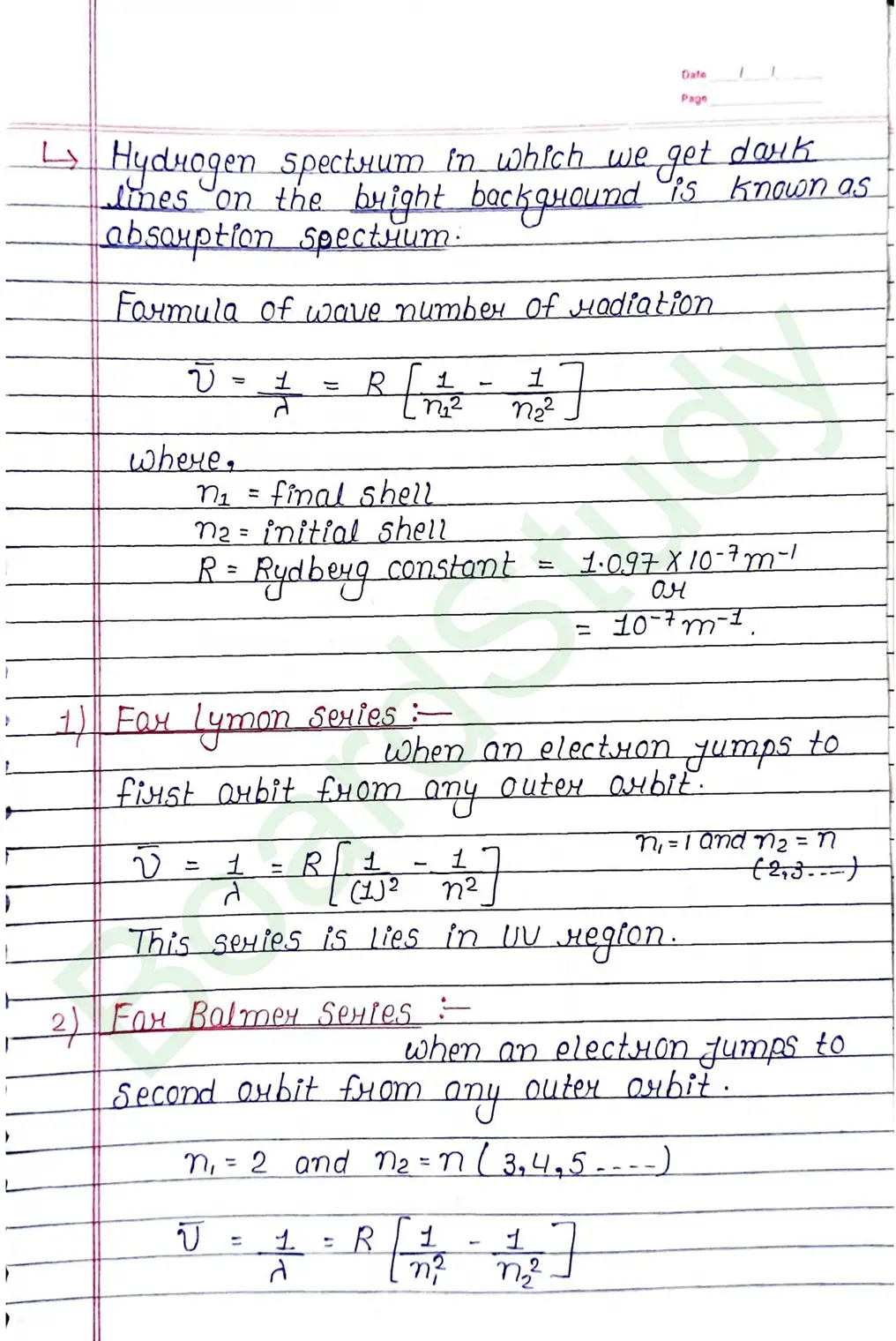
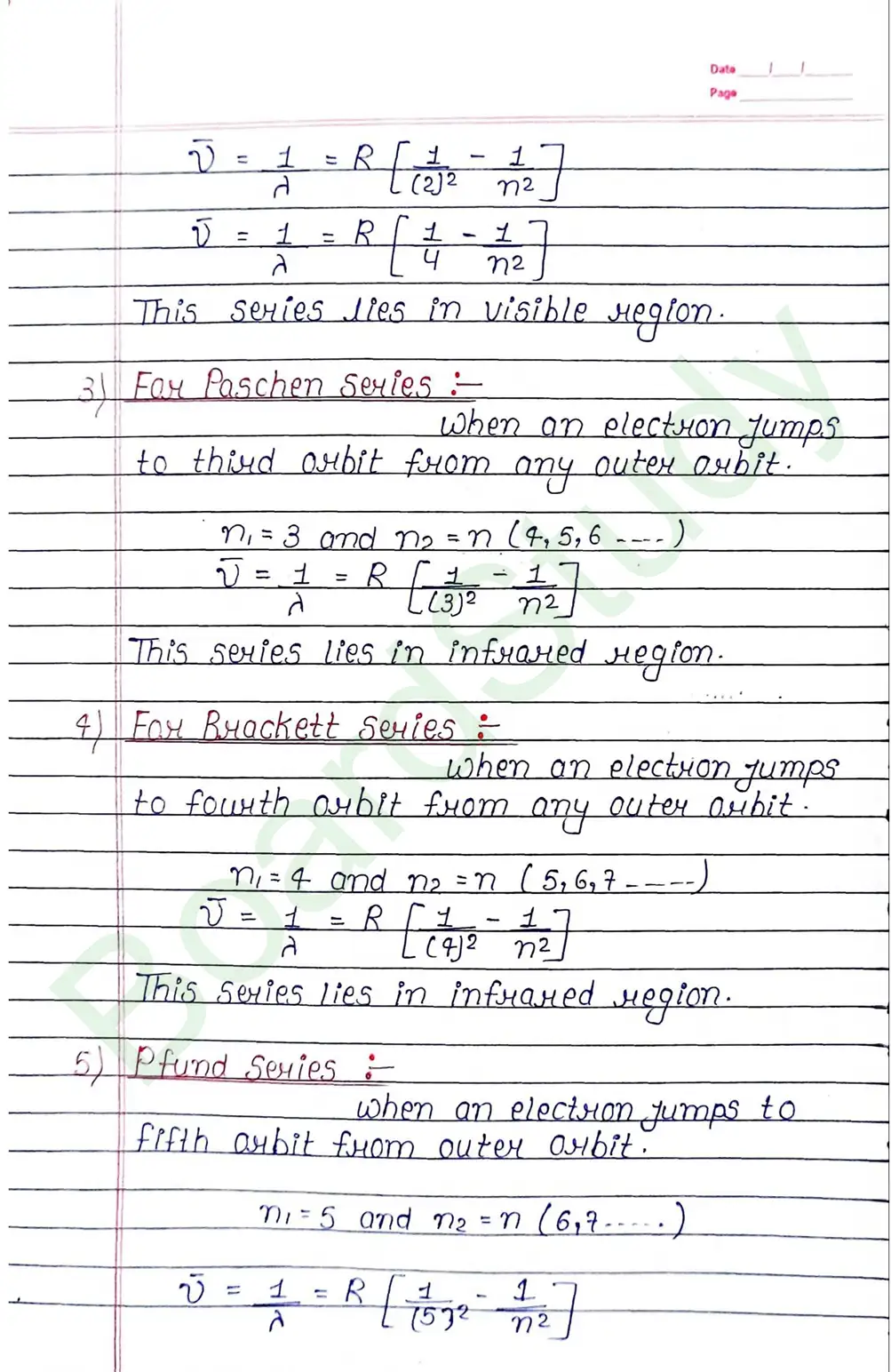
- It does not explain the structure of spectral lines.
- This model does not explain why arhital of e- are taken as circular where as eliptical arbitales are also possible.
- Bohr does say anything about relative intensities of spectrat sines
Features of Notes
- Students can use Atoms notes for last minute revision.
- In the last few days of the exam, students feel very stressed due to the pressure of the exam. Notes will be very helpful for managing the stress in the last days of the exam.
- All notes are totally free of cost and students can access notes anytime on our for totally free of cost..
- Atoms Notes PDF are created very carefully so you can rely on this notes.
Summary
| Chapter | Atoms |
| Chapter Number | 12 |
| Subject | Physics |
| Class | 12 |
| Medium | English |
FAQ
What is Atom ?
An atom is the smallest particle which can take part in all physical and chemical reactions.
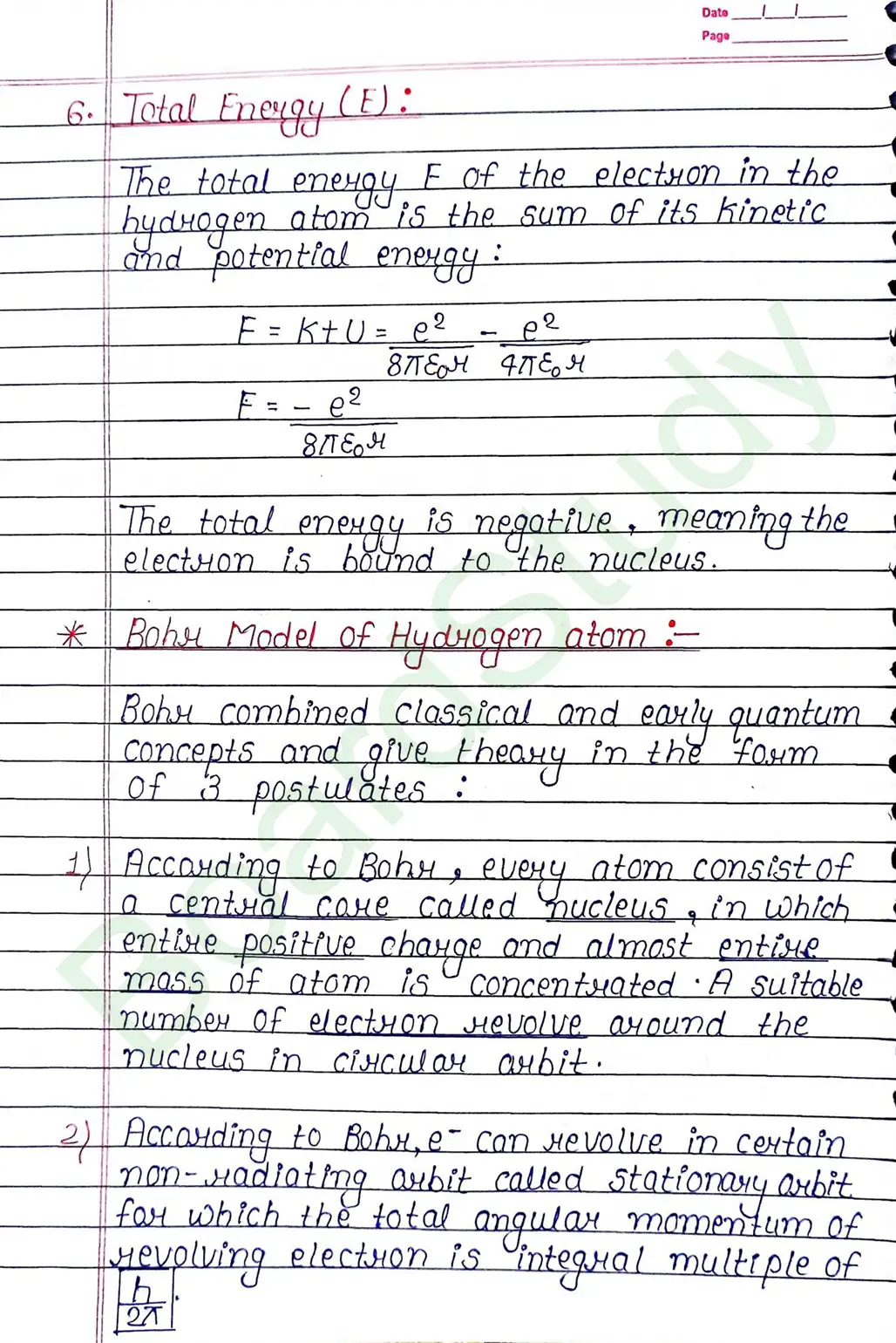
What is Centripetal Farсе ?
The electrostatic farce (Fe) between the electron and the nucleus equals the centripetal force (Fc).
Are these notes sufficient for board exam?
Atoms handwritten notes are created by topper’s and expert teacher keeping board exam in mind so you can score maximum in board exam.
Are Atoms Handwritten notes according to NCERT latest syllabus?
Yes notes are created according to the NCERT latest syllabus.
How can i download Atoms Notes PDF?
For downloading Atoms Notes PDF click on Download PDF button.
