Here we have shared class 12 Chemistry The d and f Block Elements Notes. The The d and f Block Elements notes is a best resources for students who are preparing for their board exam because it compile the entire lesson into short and includes every important topics.
With the help of The d and f Block Elements notes students can understand the chapter in a better way. Notes are prepared by very experience teachers in an organised way so students can rely on this notes for their exam preparation.
Class 12 Chemistry The d and f Block Elements Handwritten Notes






























Next Chapter: Coordination Compounds
Previous Chapter: Chemical Kinetics
Other Subjects:
Class 12 Biology Notes
Class 12 Physics Notes
Students can access this notes anytime on our website for free of cost. If you found notes helpful, you can also help your friends by sharing with them.
Key Points: The d and f Block Elements Notes PDF
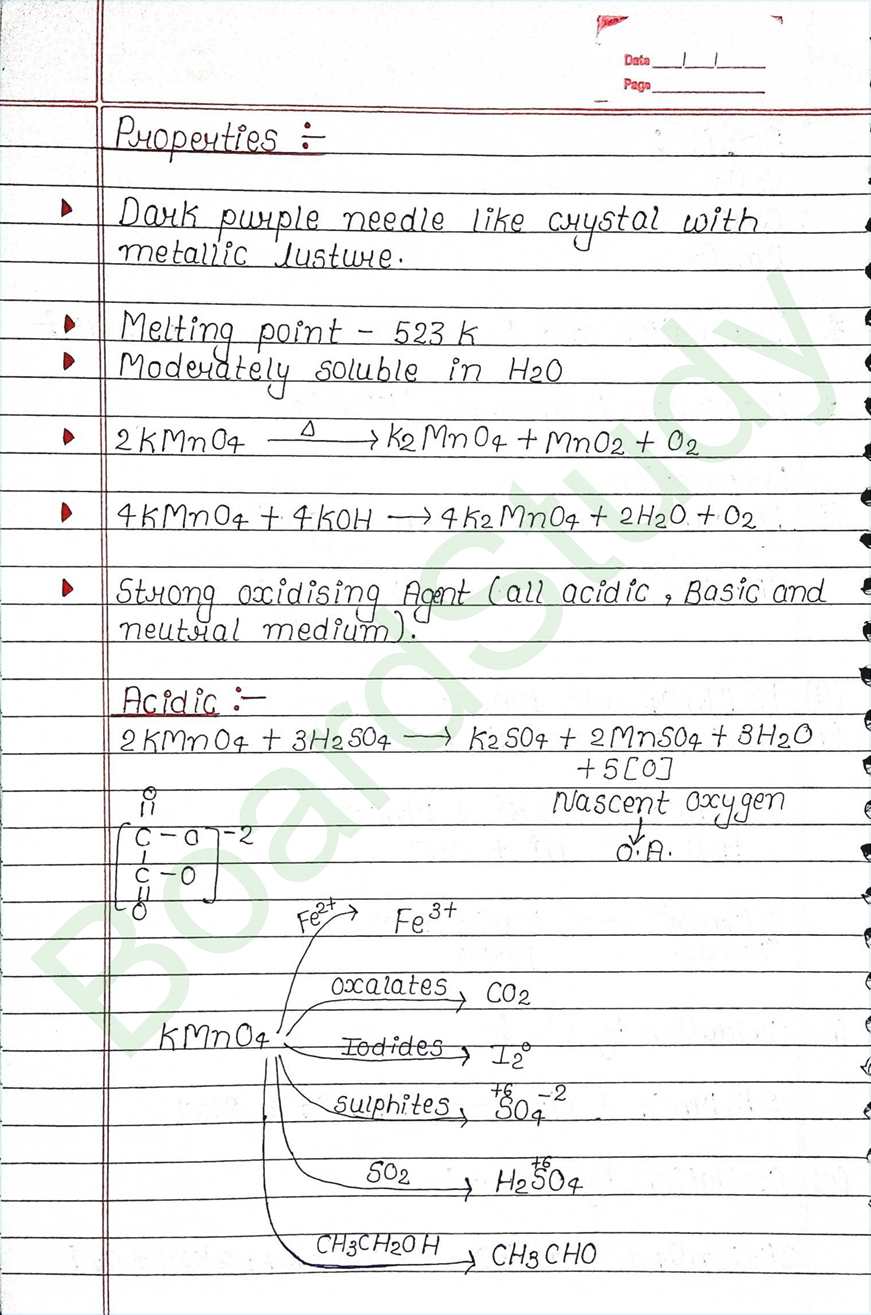
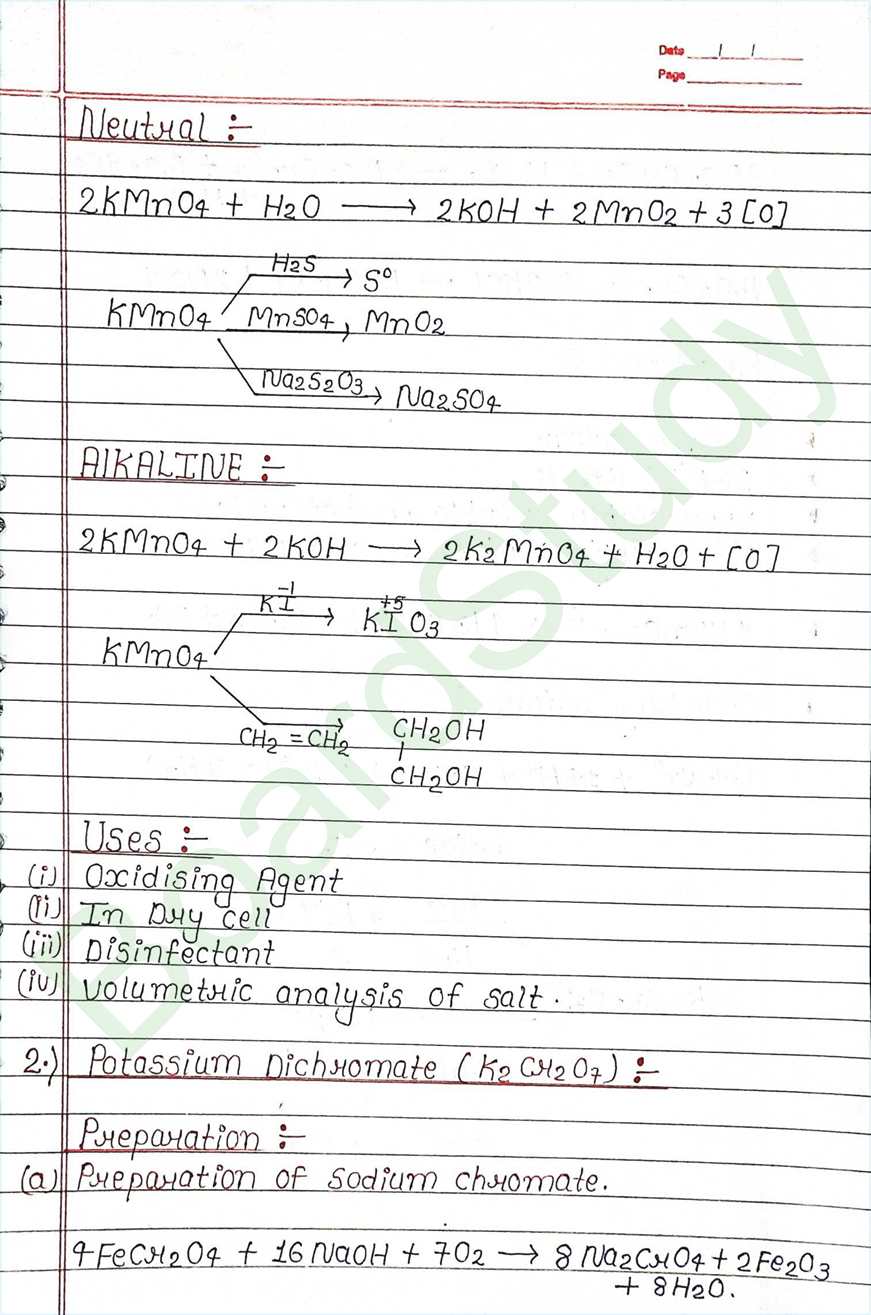
d-block-
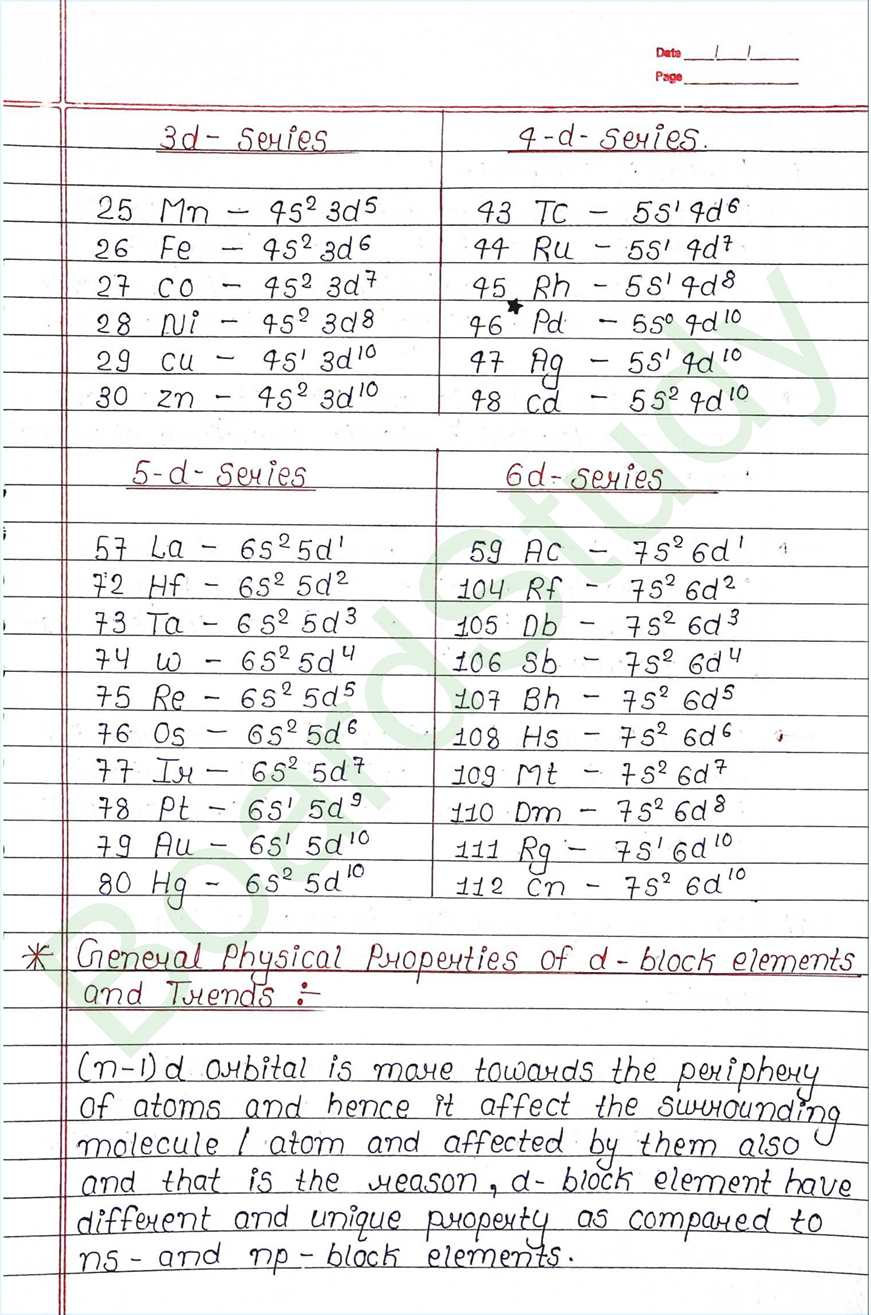
- The element that can be found from the G-3 to G-12 of modern periodic table are Called d-block elements.
- Valence e of these elements falls under the d-arbital.
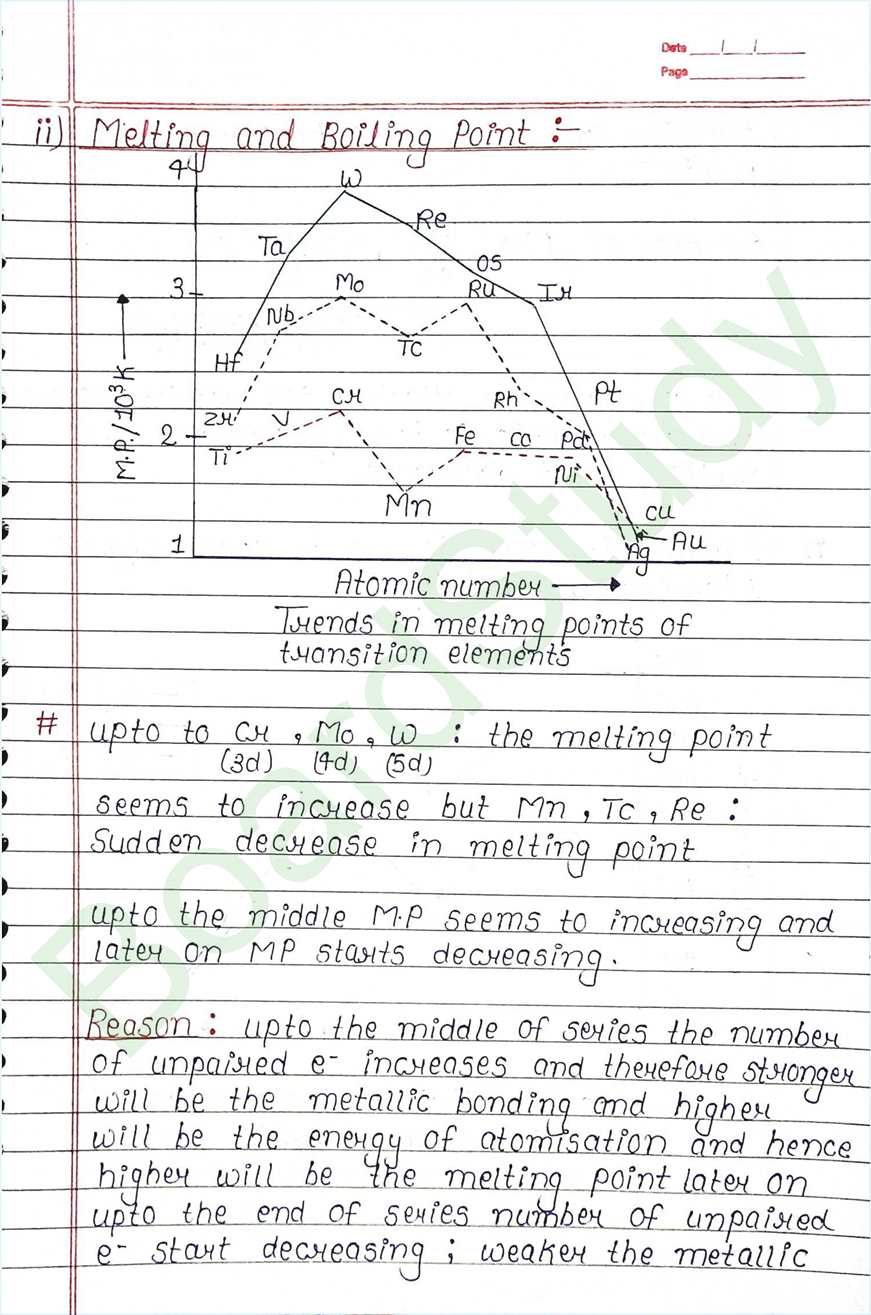
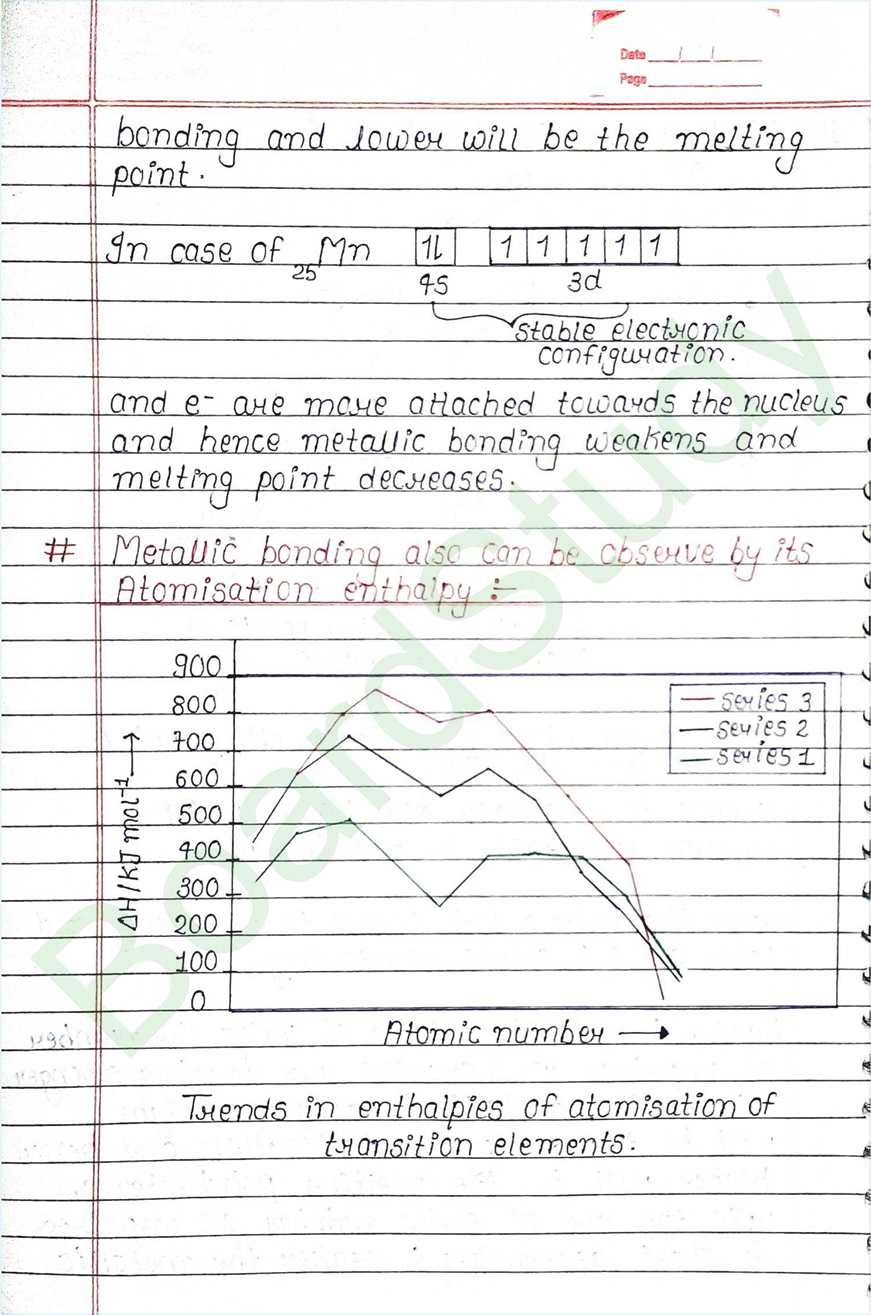
- d-block elements also called Transition element because they exibit transitional (bridging) behaviour between s-and p-block.
- In d-block elements, the valence shell has a constant number of electron, where as the number of e- in penultimate Shell goes on increasing.
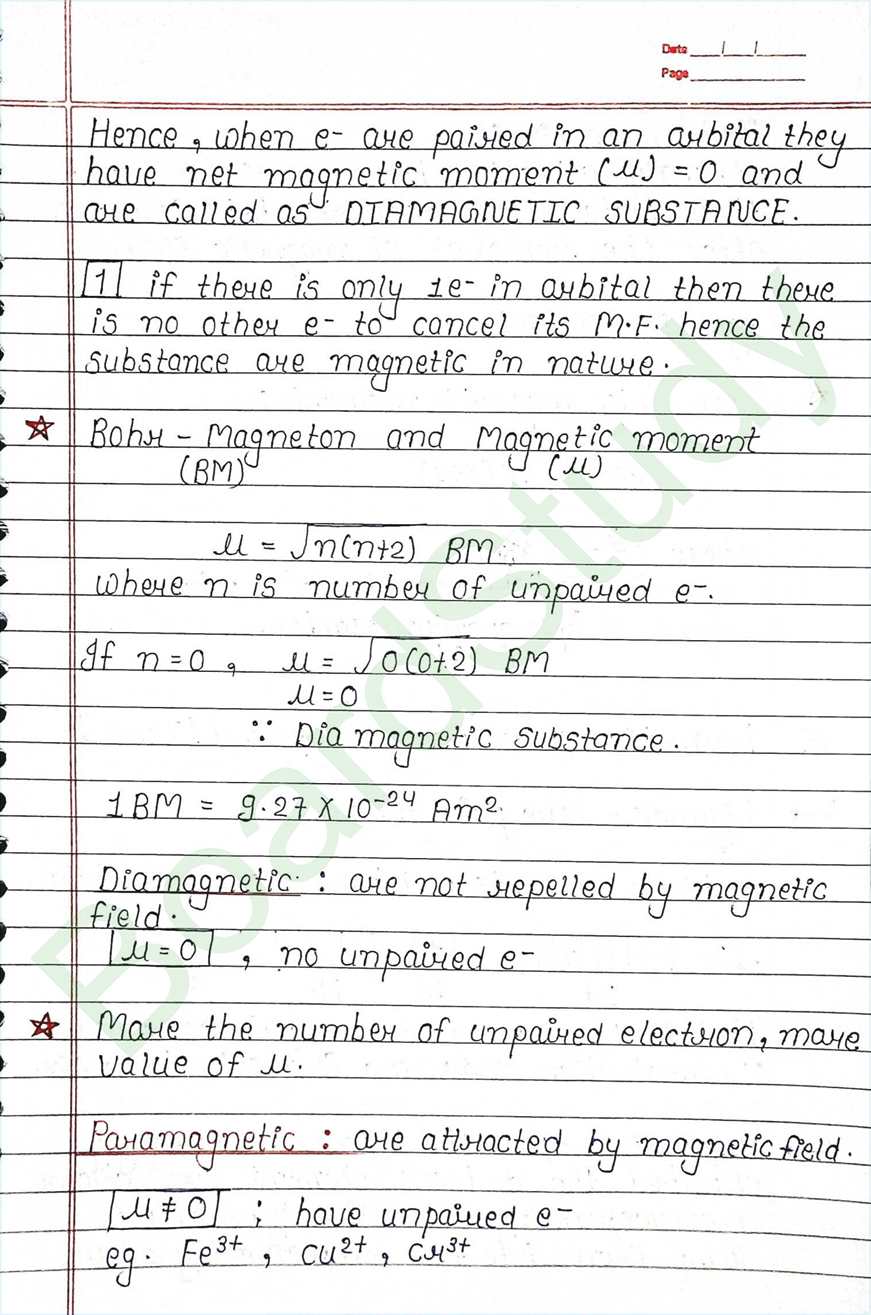
Transition elements: An element having a d subshell that is partially filled with electrons, or an element that has the ability to farm Stable cations with an incompletly filled d arbital.
f-block elements –
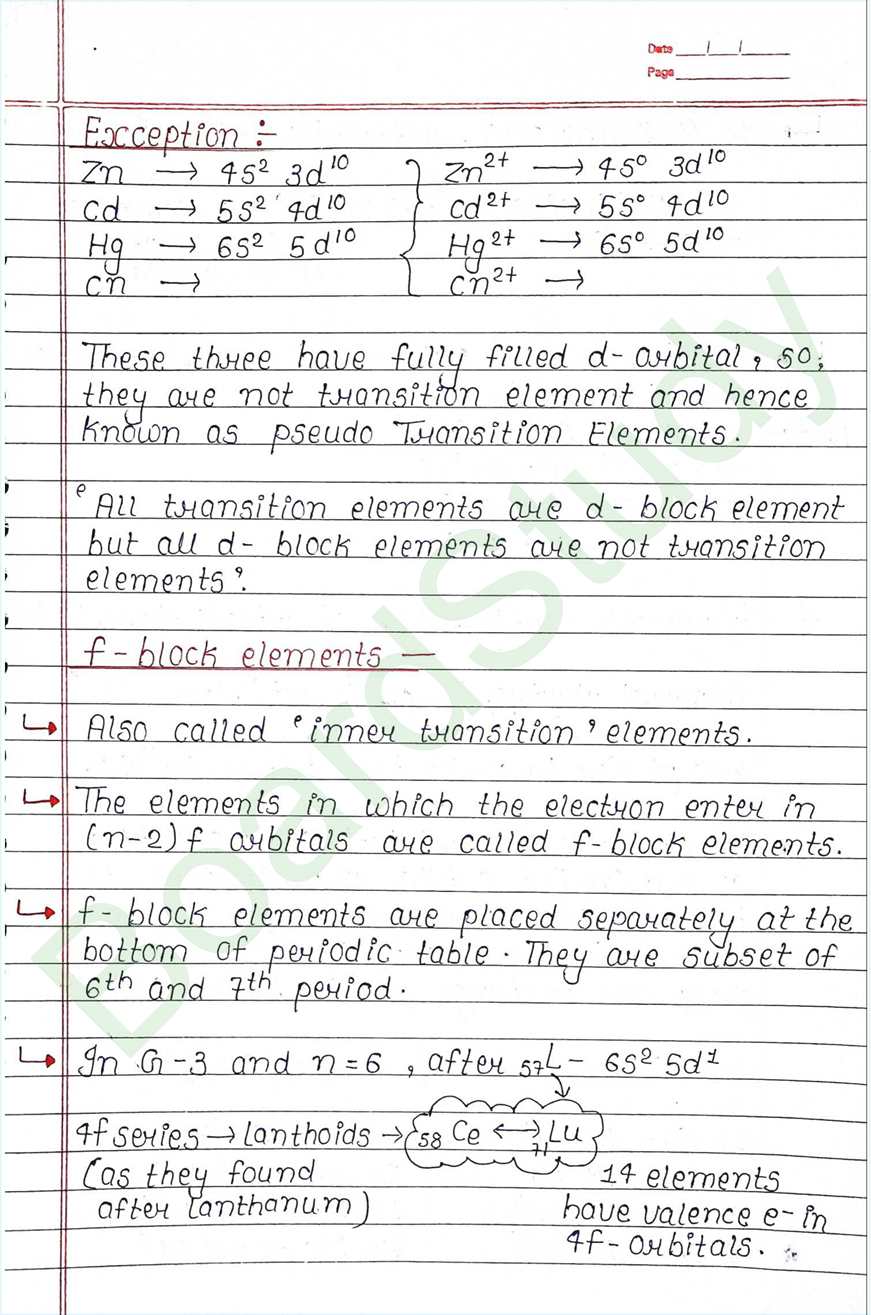
- Also called ‘inner transition’ elements.
- The elements in which the electron enter in (n-2) f arbitals are called f-block elements.
- f-block elements are placed separately at the bottom of periodic table They are subset of 6th and 7th period
Ferromagnetic :
- Strongly attracted by magnetic field and they retain the Magnetic behaviour even after the removal of magnetic field.
- eg- Fe, Co, Ni
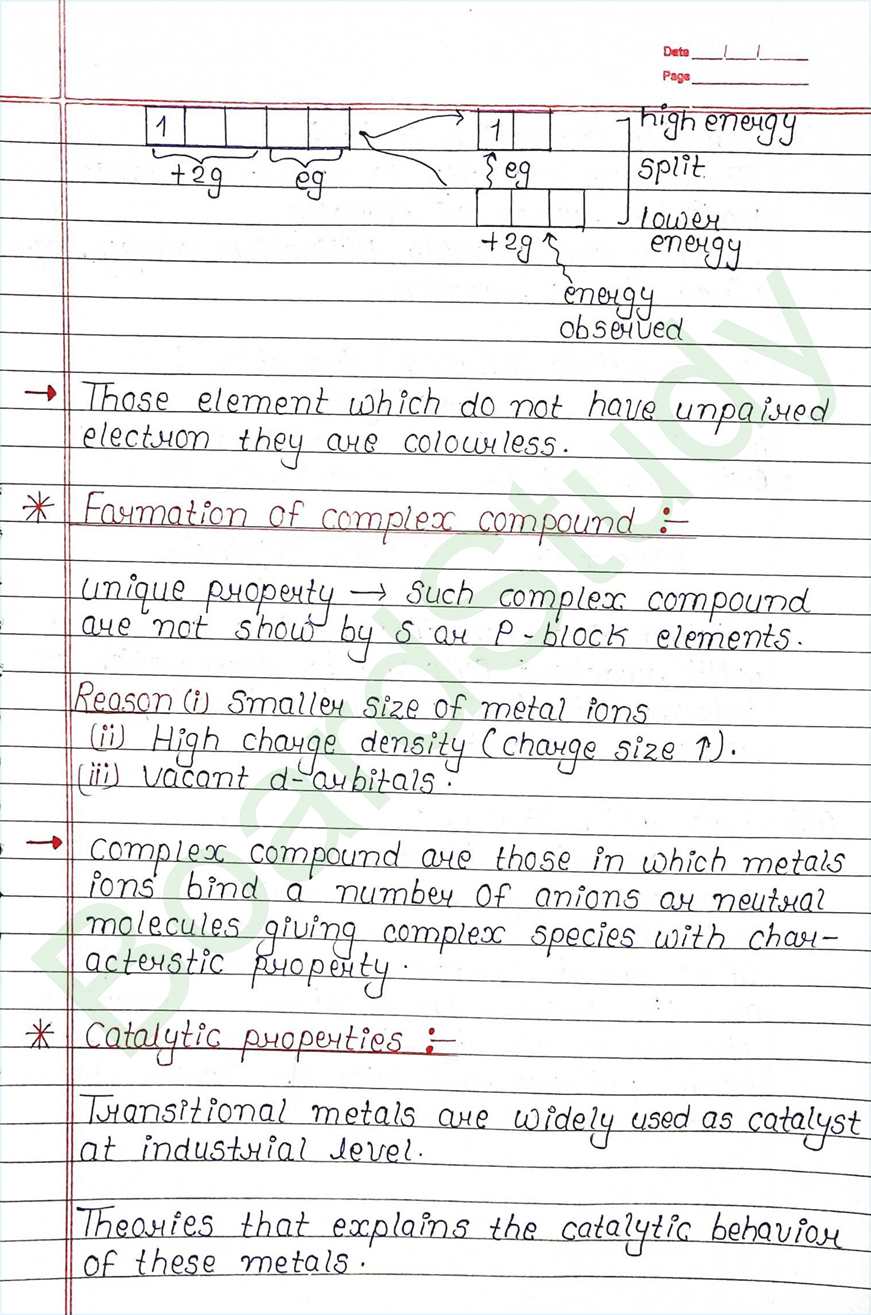
Catalytic properties :
- Transitional metals are widely used as catalyst at industrial level.
- Theories that explains the catalytic behavior of these metals.
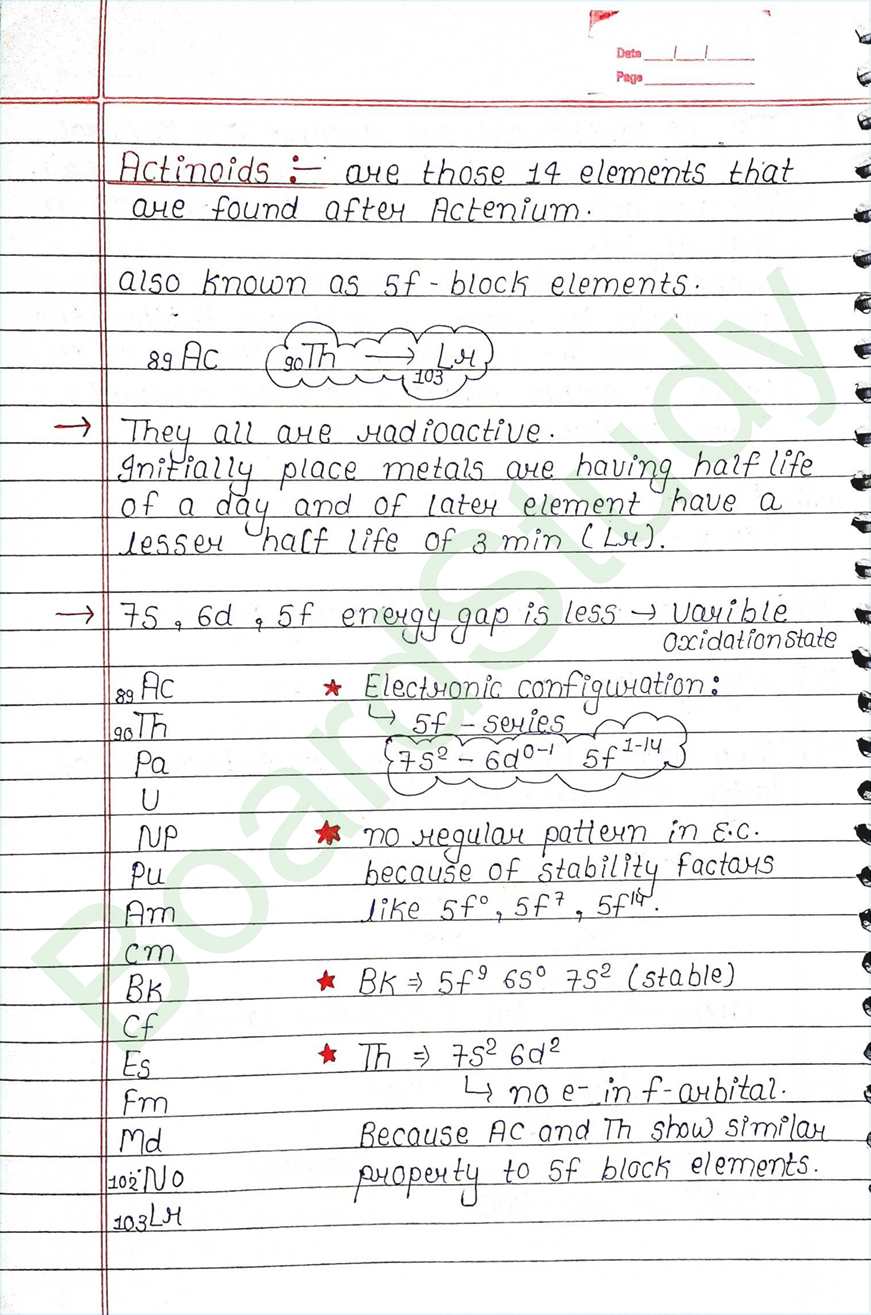
General characteristics and comparison with Lanthanoids :
- Instead of having same color (silver) all actinoid metals display the variety of structures, this variety is due to irregularities in matallic radii which are far greater than in Lanthanoids.
- The actinoids are highly reactive metals.
- The magnetic properties of the actinoids are томе соmplex than those of the Lanthanoids.
- Actinoids compound of their ions are coloured most probably due to f-f transition.
- Actinide contraction is greater than Lanthanoid contraction due to poor shielding by 5f-electrons in actinides than that of 4f-electrons in the Lanthanoids.
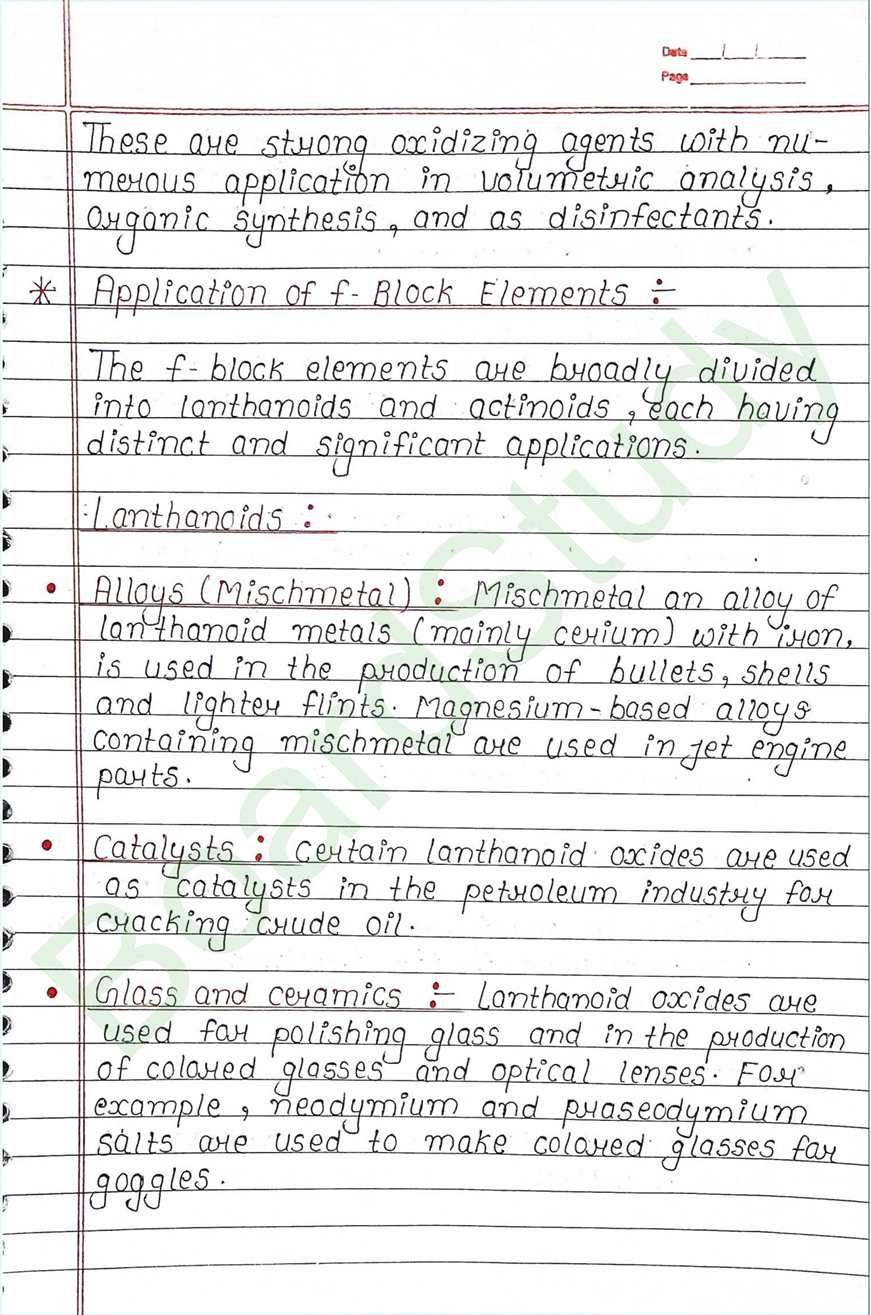
Alloy Formation
- It is a blend of metals.
- These are homogeneous solid solution.
- Alloys are formed by atoms with metallic Radii that are within 15% of each other.
- Hard and high melting point.
- Alloy of non-transition metals are brass (Cu+Zn) and bronze (Cu+Sn)
Industrial and structural Applications :-
- Iron and Steel : As the backbone of modern infrastructure, iarn and its alloy, steel, are extensively used in the construction of buildings, bridges, automobiles, and machinery. The addition of other d-block elements like chromium and nickel enhances the properties of steel, making it resistant to corrosion.
- Titanium: known for its high strength-to-weight ratio and corrosion resistance, titanium is vital in the aerospace industry for manufacturing aircraft and Spacecraft components. it is also used in medical implants due to its biocompatibility.
- Copper: An excellent conductor of electricity and heat, copper is primarily used in electrical wiring, plumbing, and in the manufacturing of various alloys like brass (with zinc) and bronze (with tin).
- Zinc : A key application of zinc is in the galvanization of iron to protect it from rusting. Zinc is also a crucial component of batteries and various alloys.
- Tungsten : with its exceptionally high melting point, tungsten is used to make filaments far incandescent light bulbs and heating elements in high-temperature furnaces.
- Chromium and Nickel :- These metals are widely used for electroplating to provide a decorative and protecting coating on other metals.
Catalytic Applications: Many transition metals and their compounds are excellent catalysts due to their ability to exhibit multiple oxidation states and form intermediate complexes.
- Iron is used as a catalyst in the Haber-Bosch process for the synthesis of ammonia.
- Nickel is a crucial catalyst in the hydrogenation of vegetable oils.
Features of Notes
- Students can use The d and f Block Elements notes for last minute revision.
- In the last few days of exam students feel very stress due to pressure of exam. Notes will be very helpful for managing the stress in the last days of exam.
- All notes are totally free of cost and students can access notes anytime on our for totally free of cost.
- The d and f Block Elements Notes PDF are created very carefully so you can rely on this notes.
Summary
| Chapter | The d and f Block Elements |
| Chapter Number | 4 |
| Subject | Chemistry |
| Class | 12 |
| Medium | English |
FAQ
What is Ferromagnetic ?
Strongly attracted by magnetic field and they retain the Magnetic behaviour even after the removal of magnetic field.
What is f-block elements ?
The elements in which the electron enter in (n-2) f arbitals are called f-block elements.
Are these notes sufficient for board exam?
The d and f Block Elements handwritten notes are created by topper’s and expert teacher keeping board exam in mind so you can score maximum in board exam.
Are The d and f Block Elements Handwritten notes according to NCERT latest syllabus?
Yes notes are created according to the NCERT latest syllabus.
How can i download The d and f Block Elements Notes PDF?
For downloading The d and f Block Elements Notes PDF click on Download PDF button.

really m bohot jyada ache notes hai…phle se jaroor buy kre