Here we have shared class 12 Chemistry Chemical Kinetics Notes. Chemical Kinetics notes is a best resources for students who are preparing for their board exam because it compile the entire lesson into short and includes every important topics.
With the help of Chemical Kinetics notes students can understand the chapter in a better way. Notes are prepared by very experience teachers in an organised way so students can rely on this notes for their exam preparation.
Class 12 Chemistry Chemical Kinetics Handwritten Notes


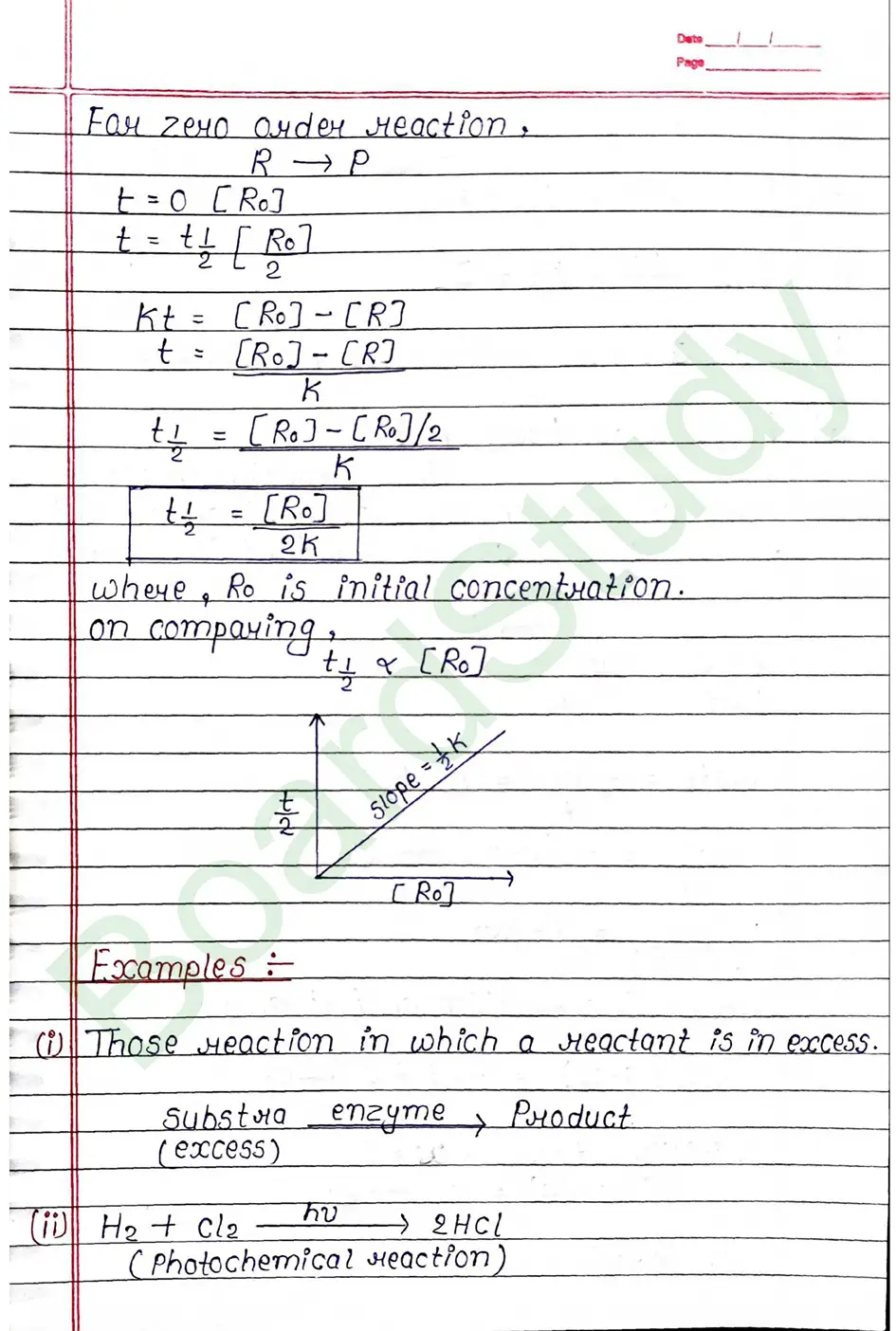
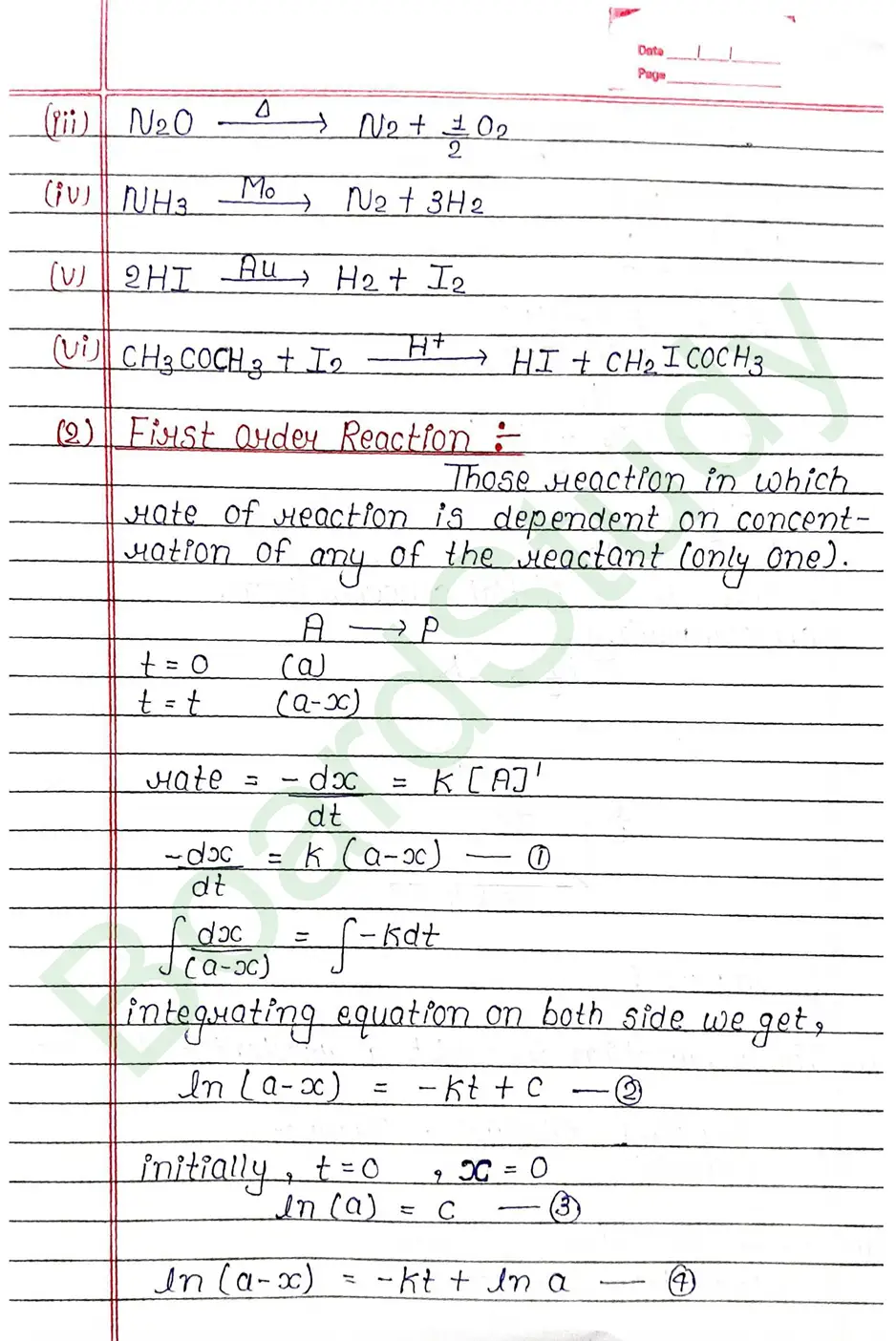
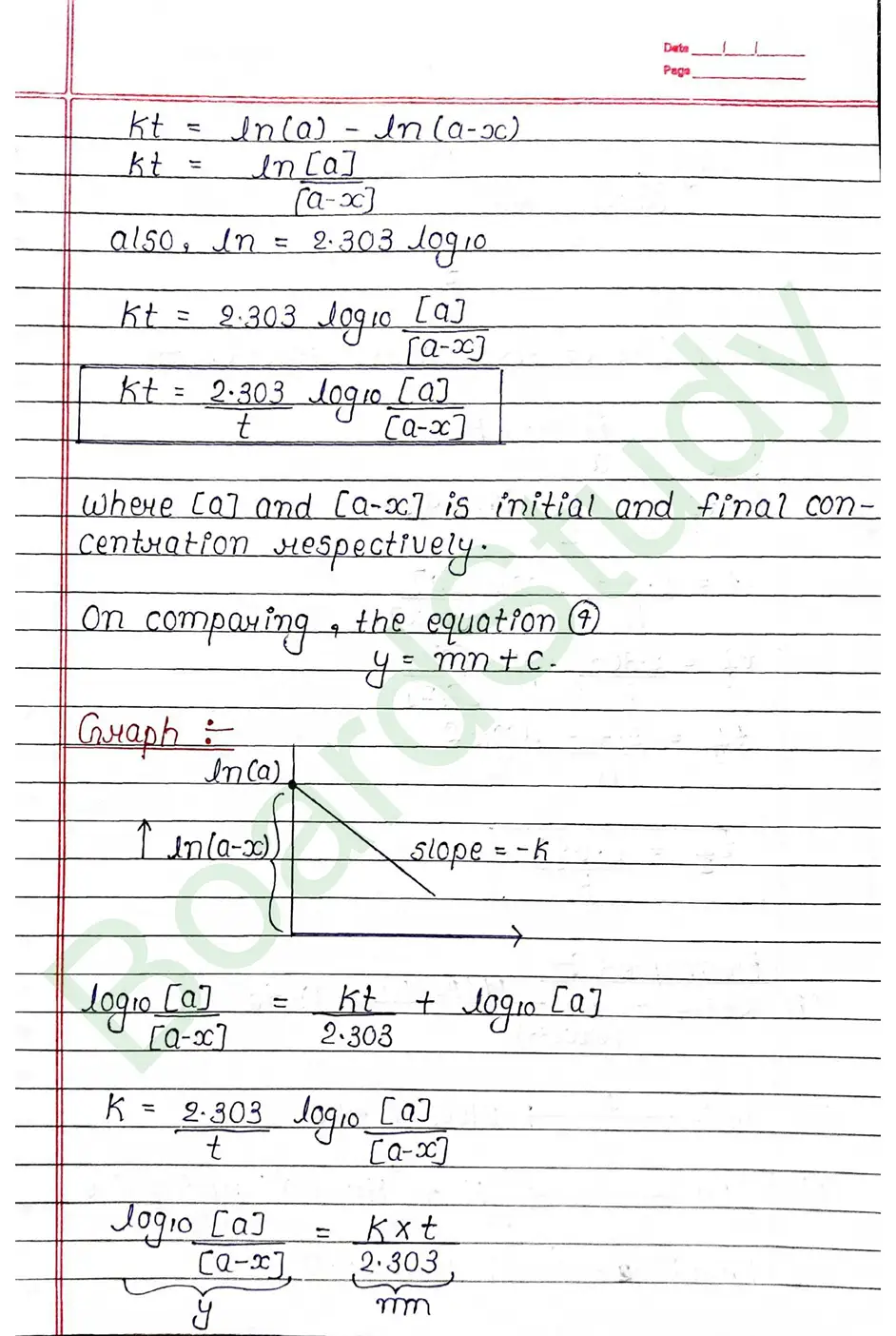
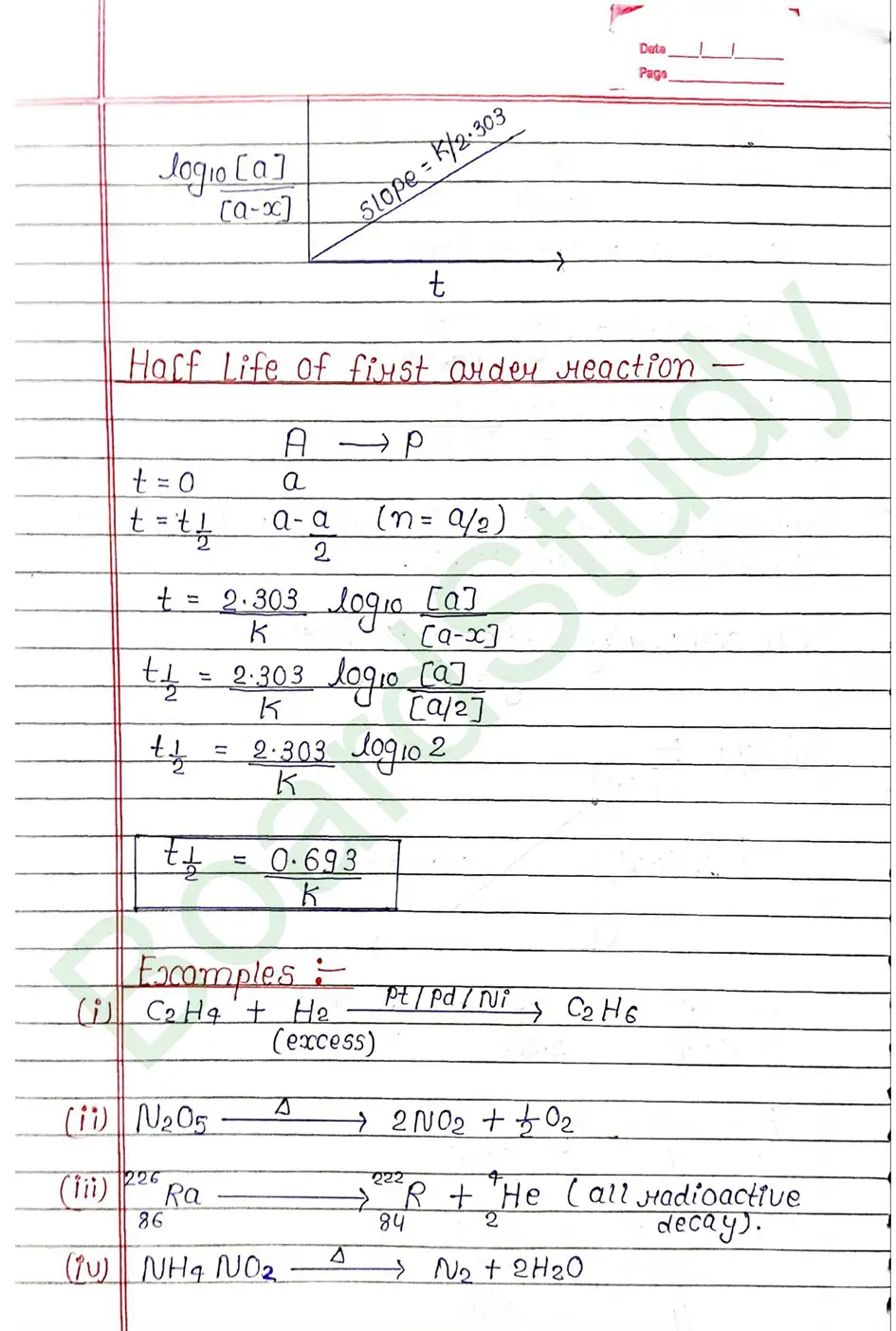

Next Chapter: The d & f Block Elements
Previous Chapter: Electrochemistry
Other Subjects:
Class 12 Biology Notes
Class 12 Physics Notes
Students can access this notes anytime on our website for free of cost. If you found notes helpful, you can also help your friends by sharing with them.
Key Points: Chemical Kinetics Notes PDF
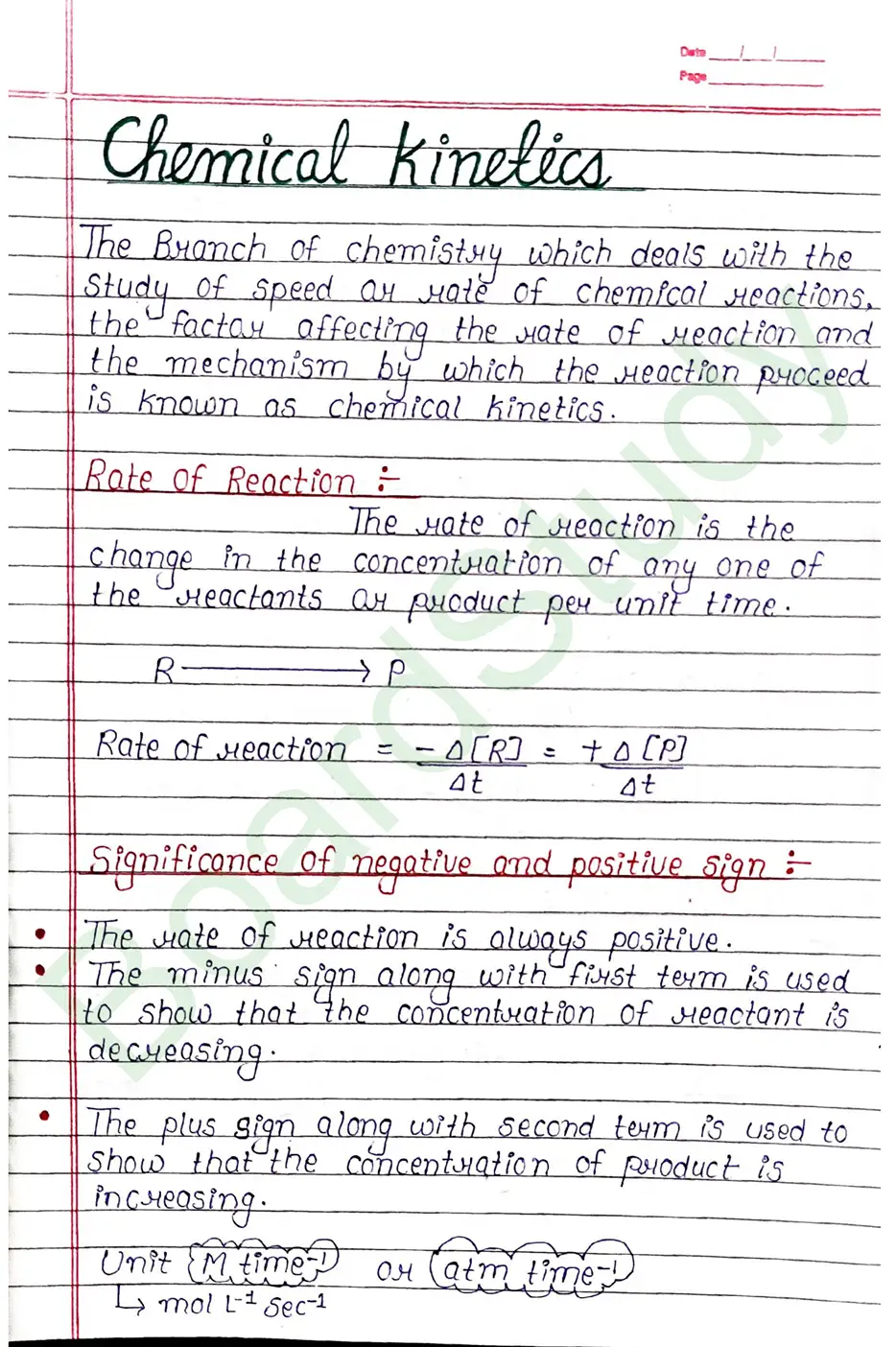
Chemical Kinetics
The Branch of chemistry which deals with the Study of speed or rate of chemical reactions, the factar affecting the mate of reaction and the mechanism by which the reaction proceed is known as chemical kinetics.
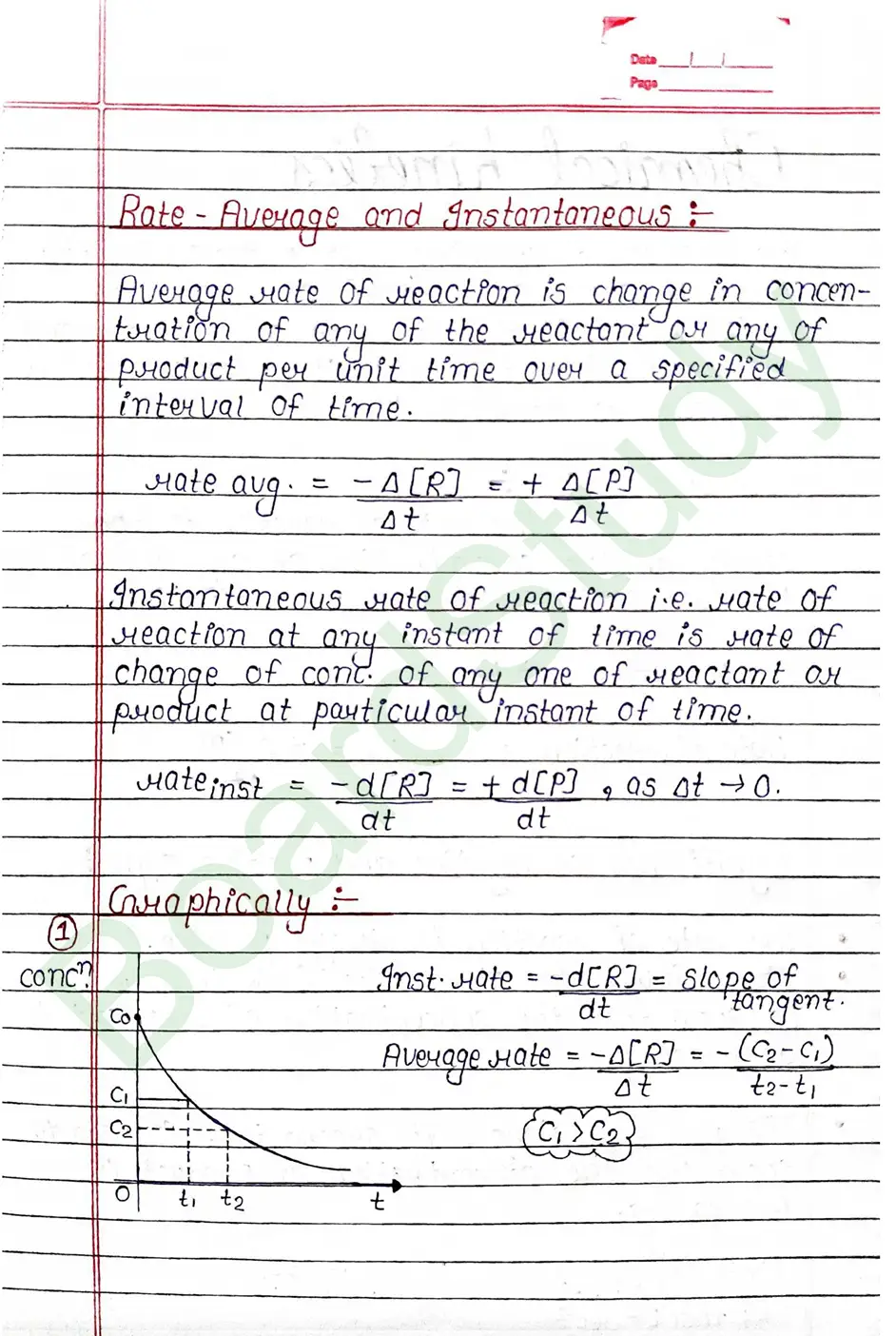
Rate of Reaction
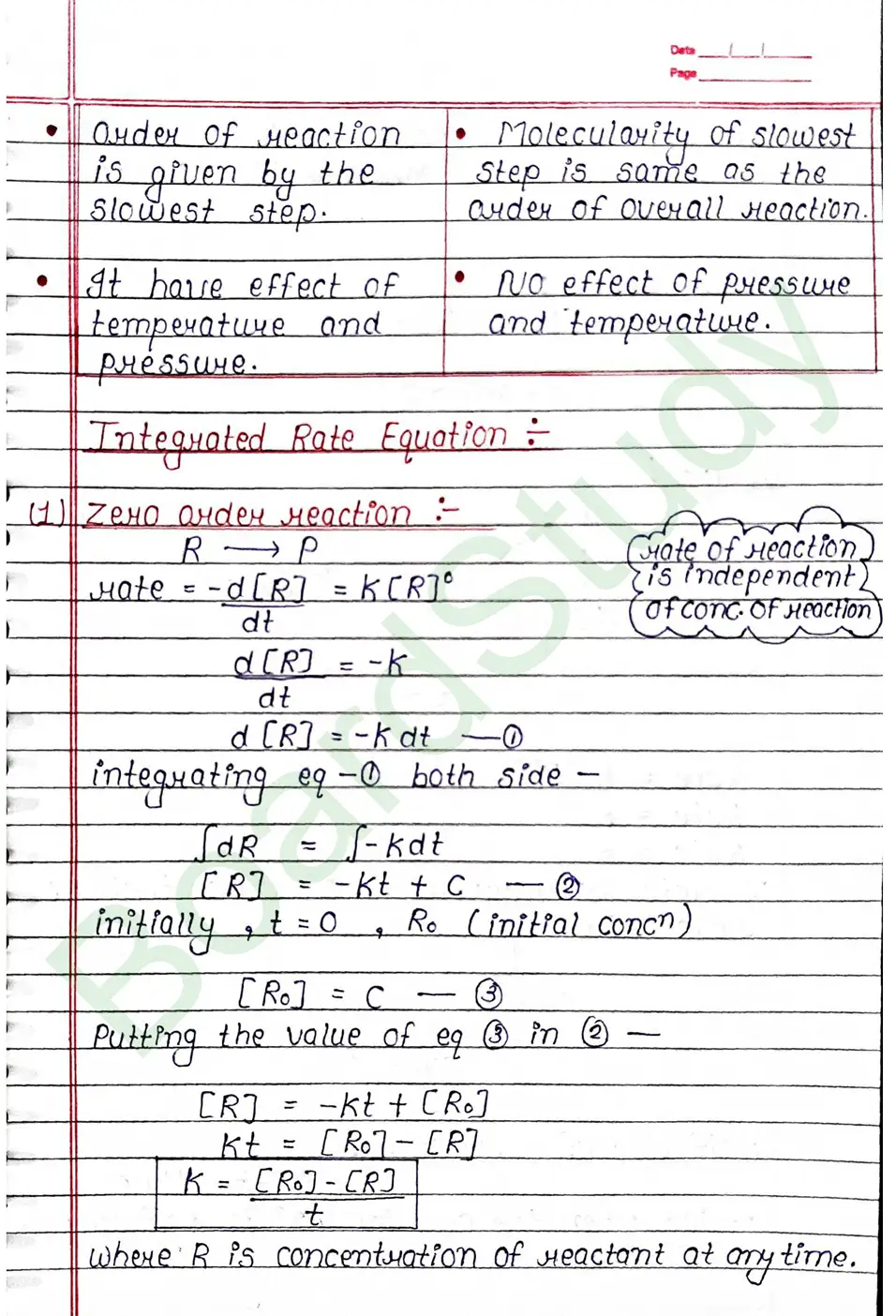
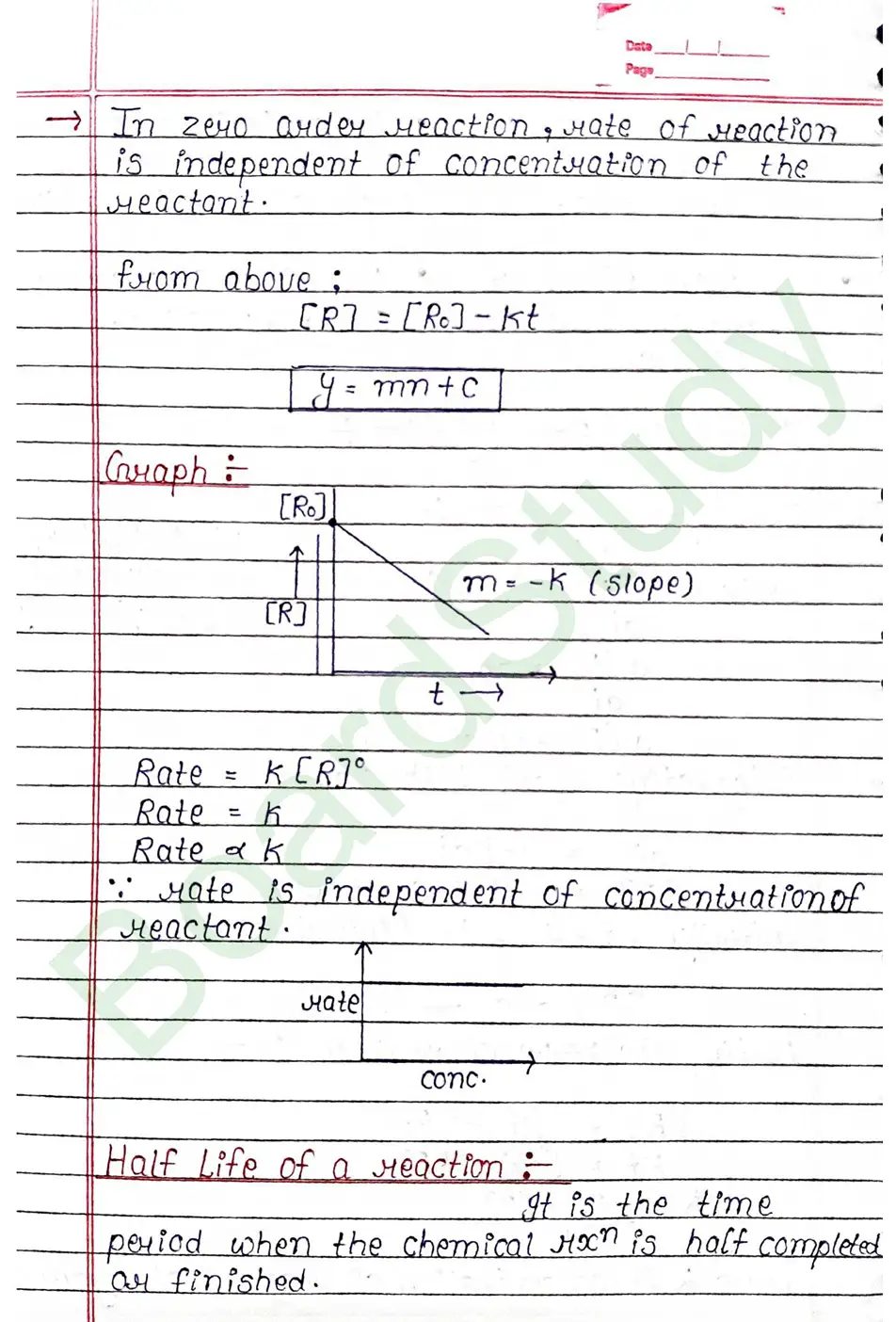
The rate of reaction is the change in the concentration of any one of the reactants or product per unit time.
Rate Law
It states that rate of chemical reaction is directly proportional to product of concentration of reactant to the some power, which may or may not be equal to Mespective stoichiometric coefficient.
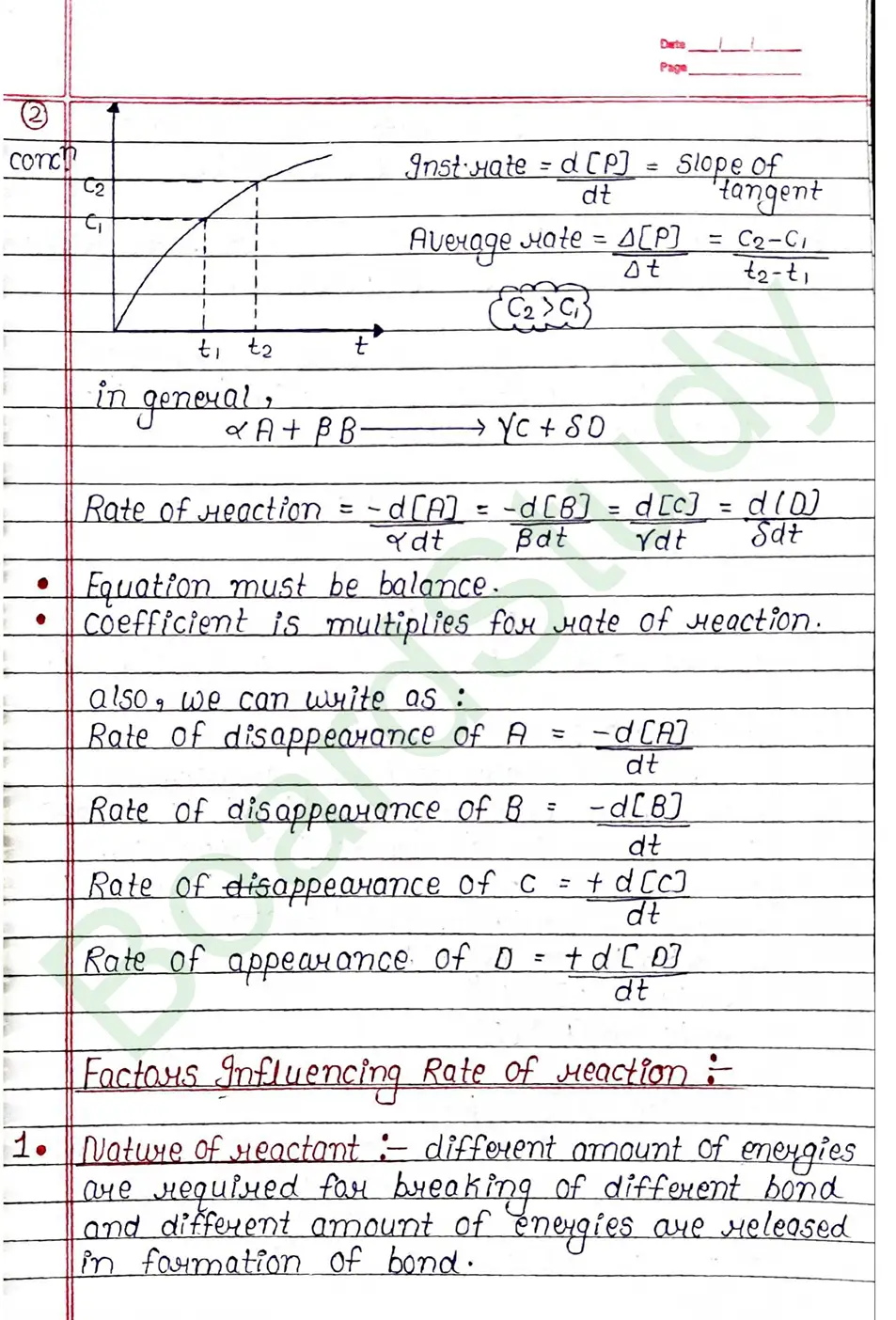
Factors Influencing Rate of reaction :
- Nature of reactant :- different amount of energies are required for breaking of different bond and different amount of energies are released in formation of bond.
- Concentration : Greater the reactant’s concentration, faster the reaction.
- Temperature: The rate of reaction increases on increasing of temperature.
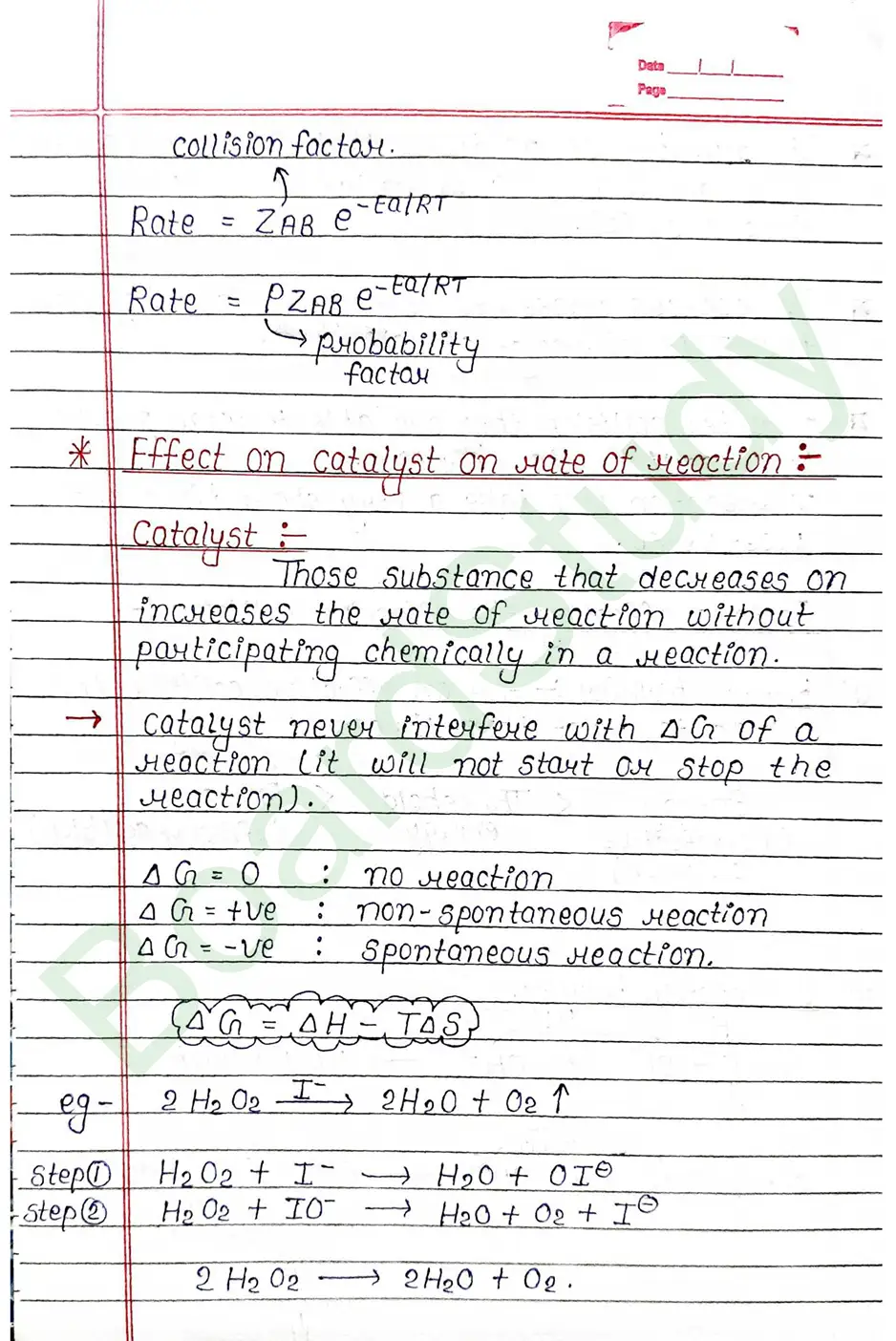
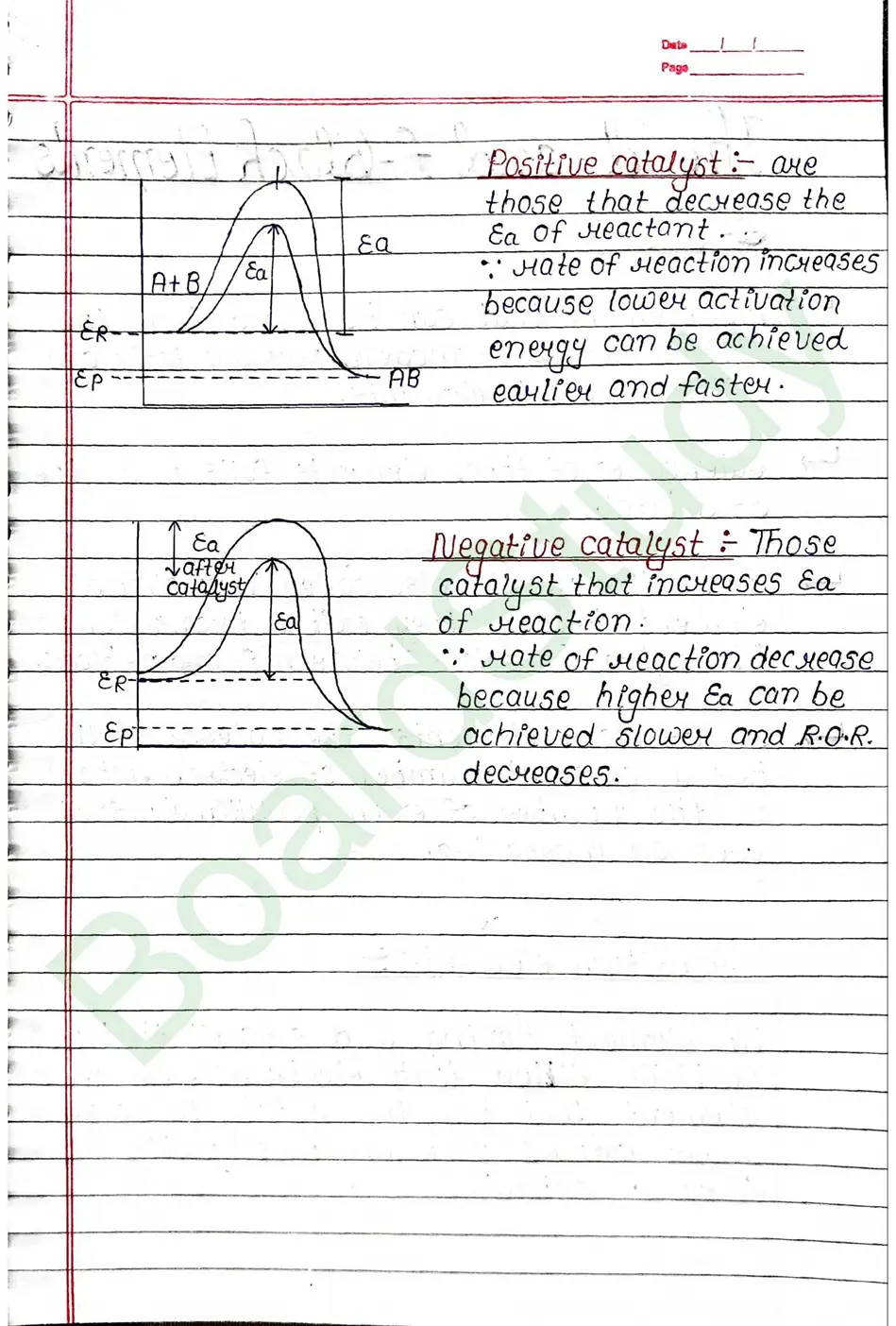
- Presence of catalyst : A catalyst generally increase the speed of a reaction without being consumed in reaction.
- Surface area of reactant : greater the surface area, faster the reaction.
- Presence of Madiation: some reaction’s rate increases with increase in intensity of light.
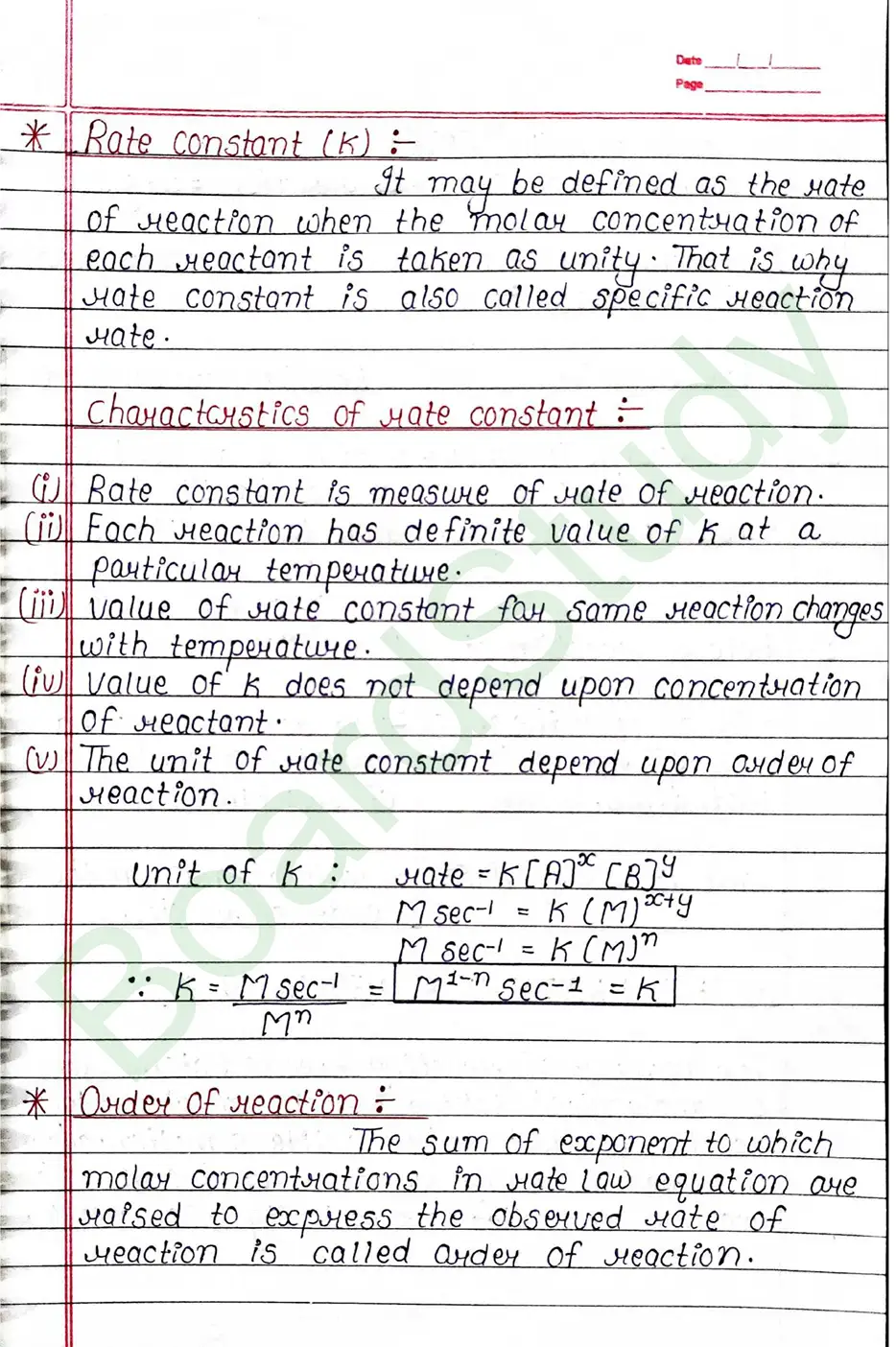
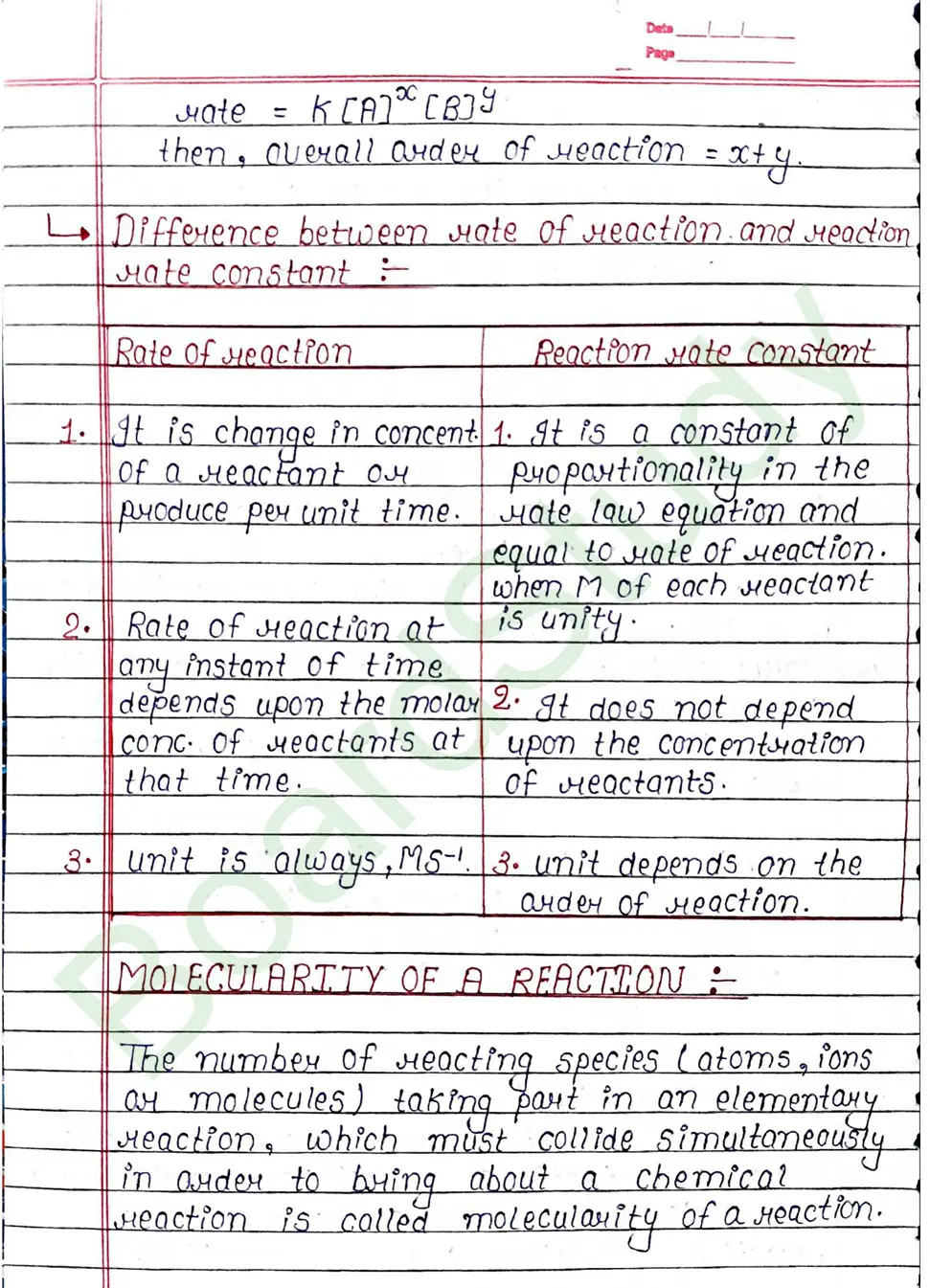
Rate Constant (K)
It may be defined as the rate of reaction when the molar concentration of each reactant is taken as unity. That is why rate constant is also called specific reaction rate.
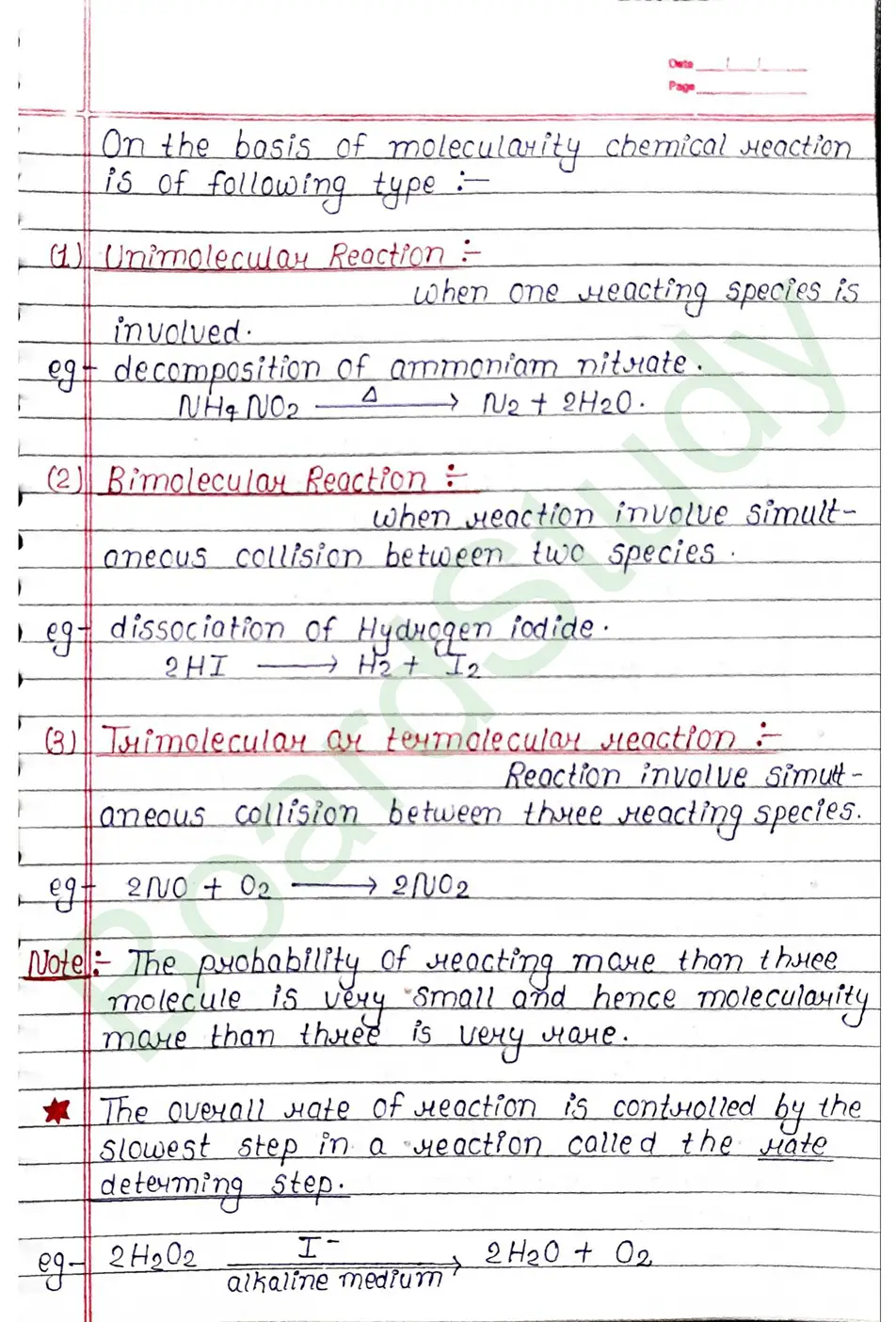
Molecularity of a Reaction
The number of reacting species (atoms, ions ar molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reaction is called molecularity of a reaction.
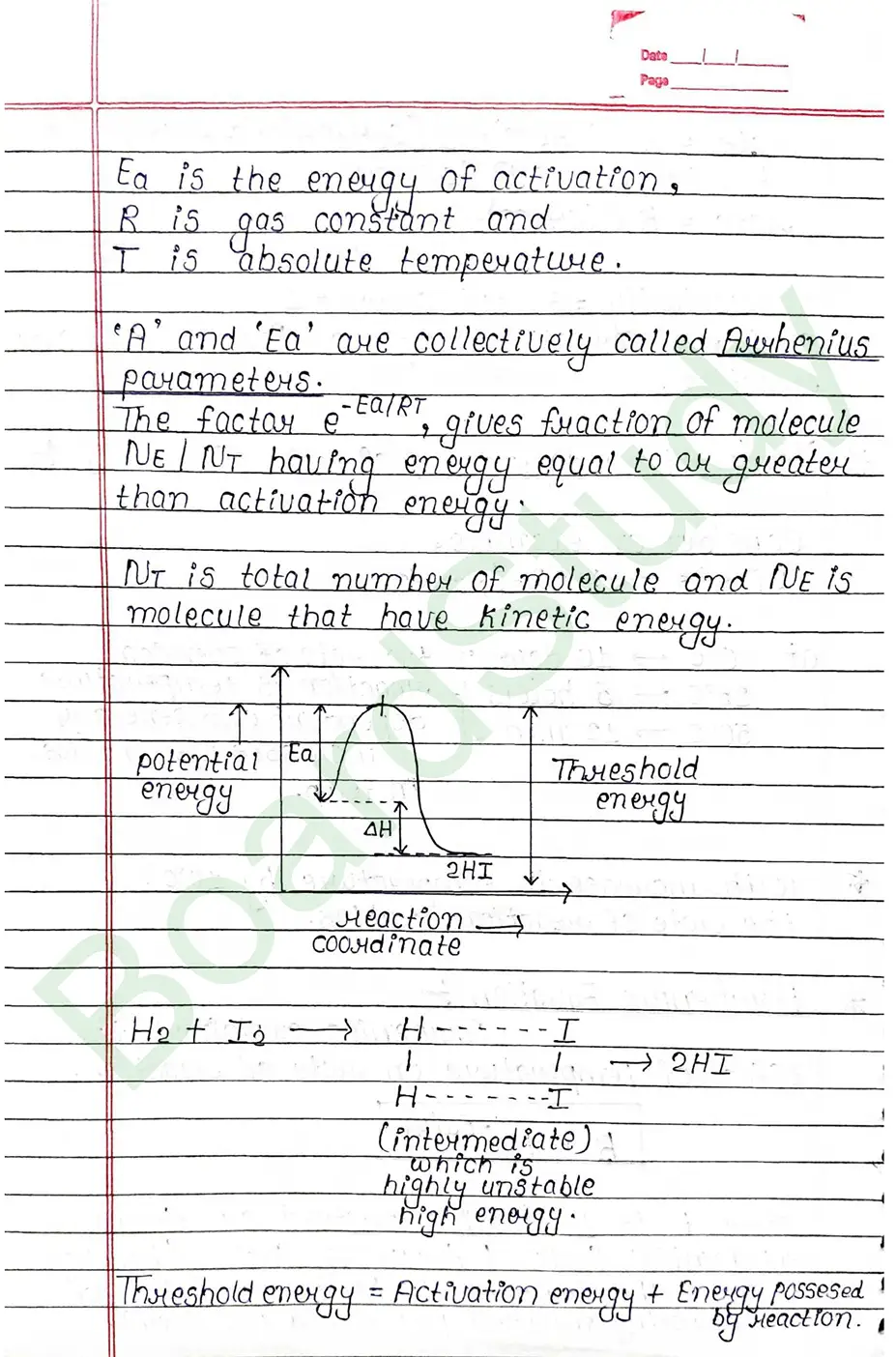
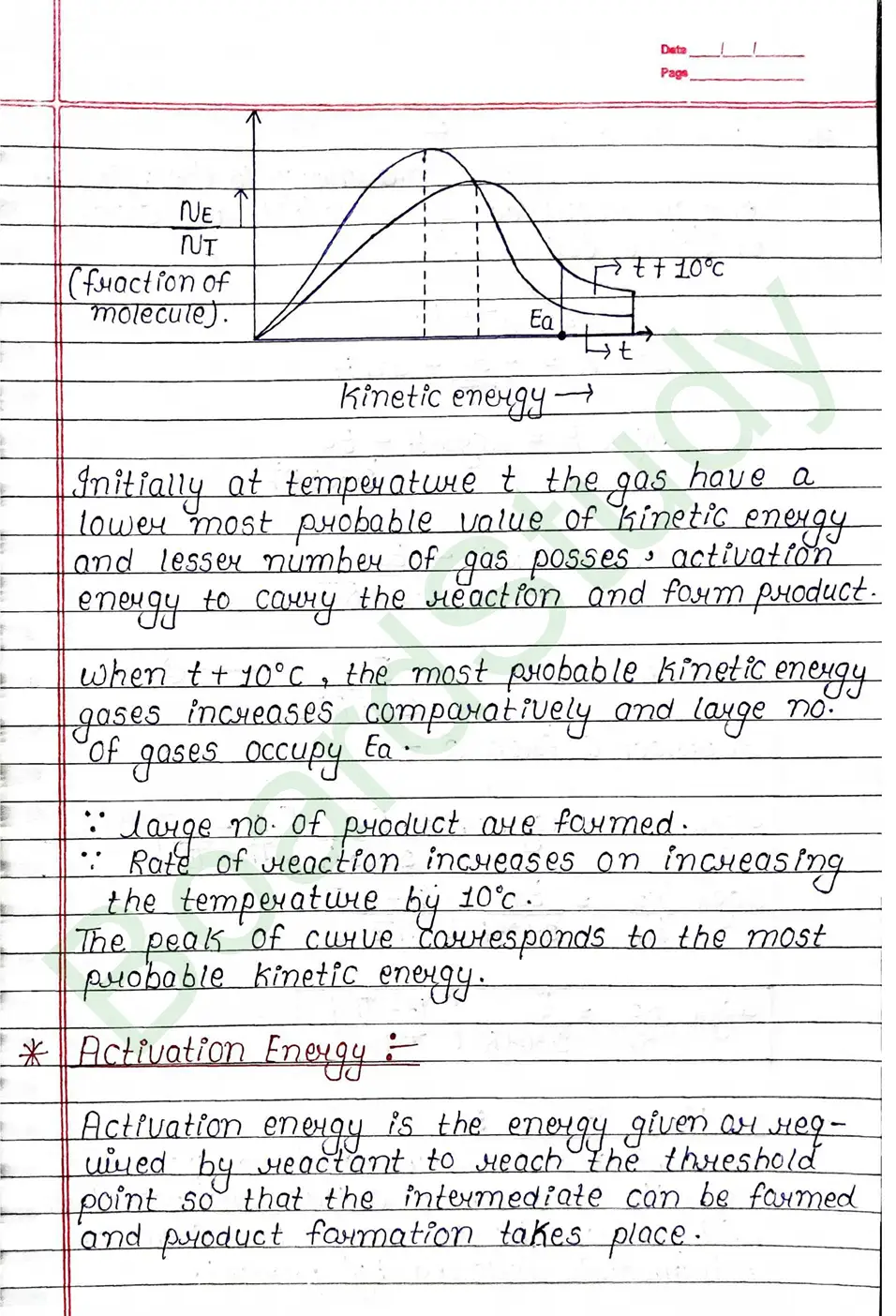
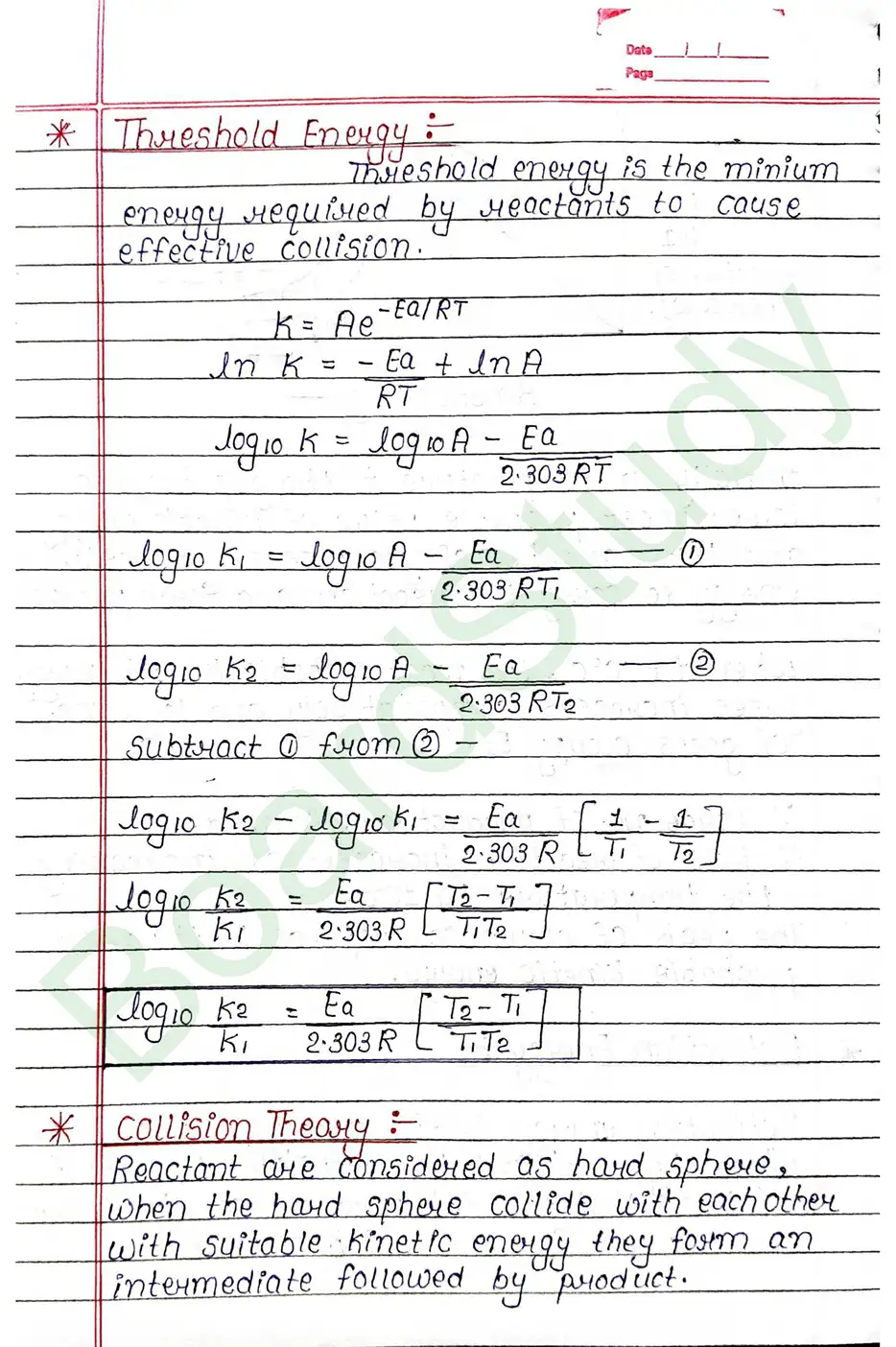
Activation energy
Activation energy is the energy given or required by reactant to reach the threshold point so that the intermediate can be farmed and product farmation takes place.
Collision Theory
Reactant are considered as hard sphere, when the hard sphere collide with each other with suitable kinetic energy they form an intermediate followed by product.
- The number of particles that collide per second in a given volume is called as collision frequency (z).
- In gaseous phase, for binary collision system the z is in range of 10^25 – 10^28.
- If after collision they are able to farm product then collision is effective.
- reaction will take a very short time and vice-versa.
Catalyst: Those substance that decreases on increases the rate of reaction without participating chemically in a meaction.
Features of Notes
- Students can use Chemical Kinetics notes for last minute revision.
- In the last few days of exam students feel very stress due to pressure of exam. Notes will be very helpful for managing the stress in the last days of exam.
- All notes are totally free of cost and students can access notes anytime on our for totally free of cost.
- Chemical Kinetics Notes PDF are created very carefully so you can rely on this notes.
Summary
| Chapter | Chemical Kinetics |
| Chapter Number | 3 |
| Subject | Chemistry |
| Class | 12 |
| Medium | English |
FAQ
What is Catalyst ?
Those substance that decreases on increases the rate of reaction without participating chemically in a meaction.
What is Arrhenius Equation ?
Arrhenius explained the effect of temperature on Mate of reaction
Are these notes sufficient for board exam?
Chemical Kinetics handwritten notes are created by topper’s and expert teacher keeping board exam in mind so you can score maximum in board exam.
Are Chemical Kinetics Handwritten notes according to NCERT latest syllabus?
Yes notes are created according to the NCERT latest syllabus.
How can i download Chemical Kinetics Notes PDF?
For downloading Chemical Kinetics Notes PDF click on Download PDF button.

Can I get pdf chemical kinetics
I want to notes