Here we have shared class 12 Chemistry Electrochemistry Notes. Electrochemistry notes is a best resources for students who are preparing for their board exam because it compile the entire lesson into short and includes every important topics.
With the help of Electrochemistry notes students can understand the chapter in a better way. Notes are prepared by very experience teachers in an organised way so students can rely on this notes for their exam preparation.
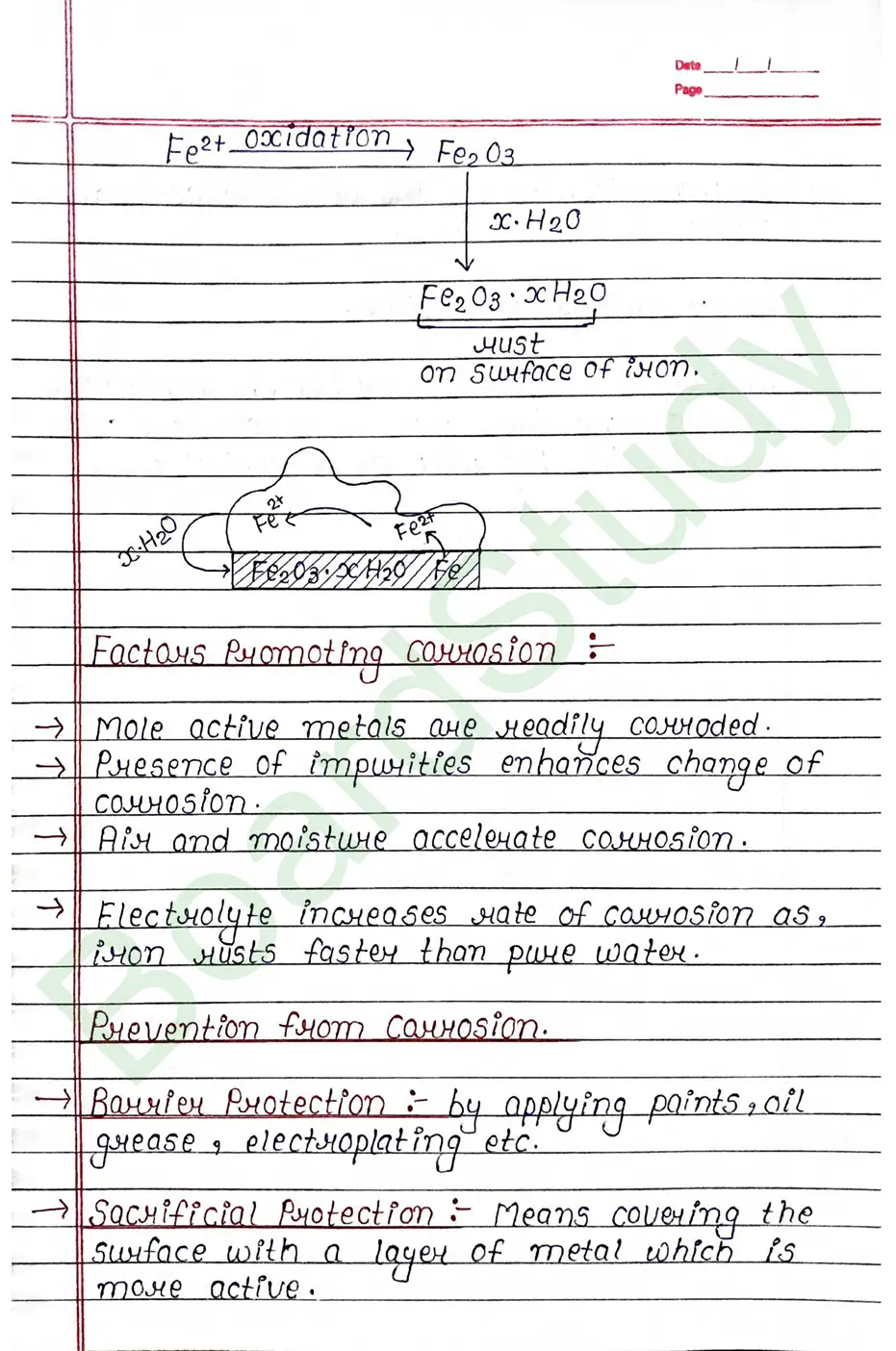
Class 12 Chemistry Electrochemistry Handwritten Notes









Next Chapter: Chemical Kinetics
Previous Chapter: Solutions
Other Subjects:
Class 12 Biology Notes
Class 12 Physics Notes
Students can access this notes anytime on our website for free of cost. If you found notes helpful, you can also help your friends by sharing with them.
Key Points: Electrochemistry Notes PDF
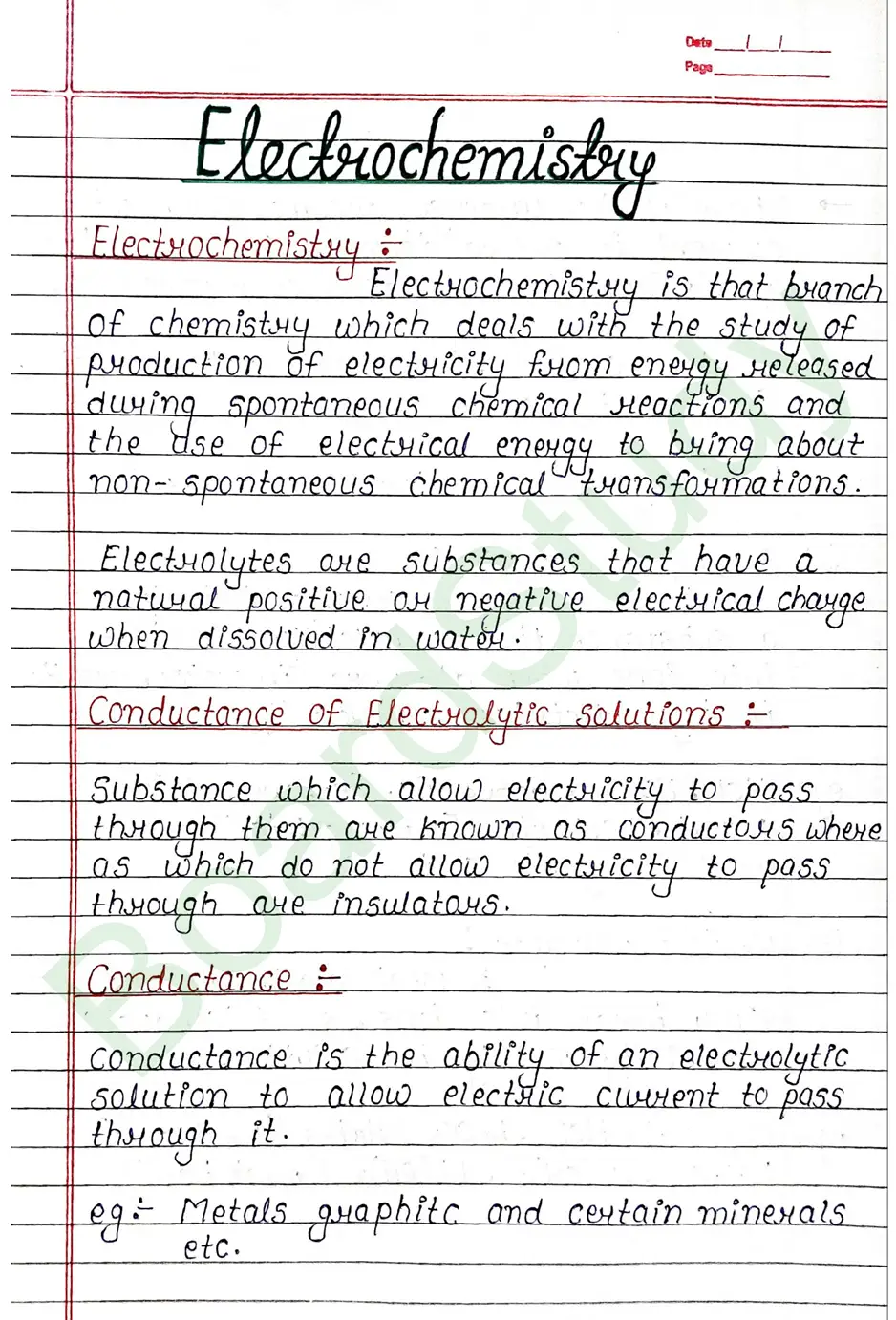
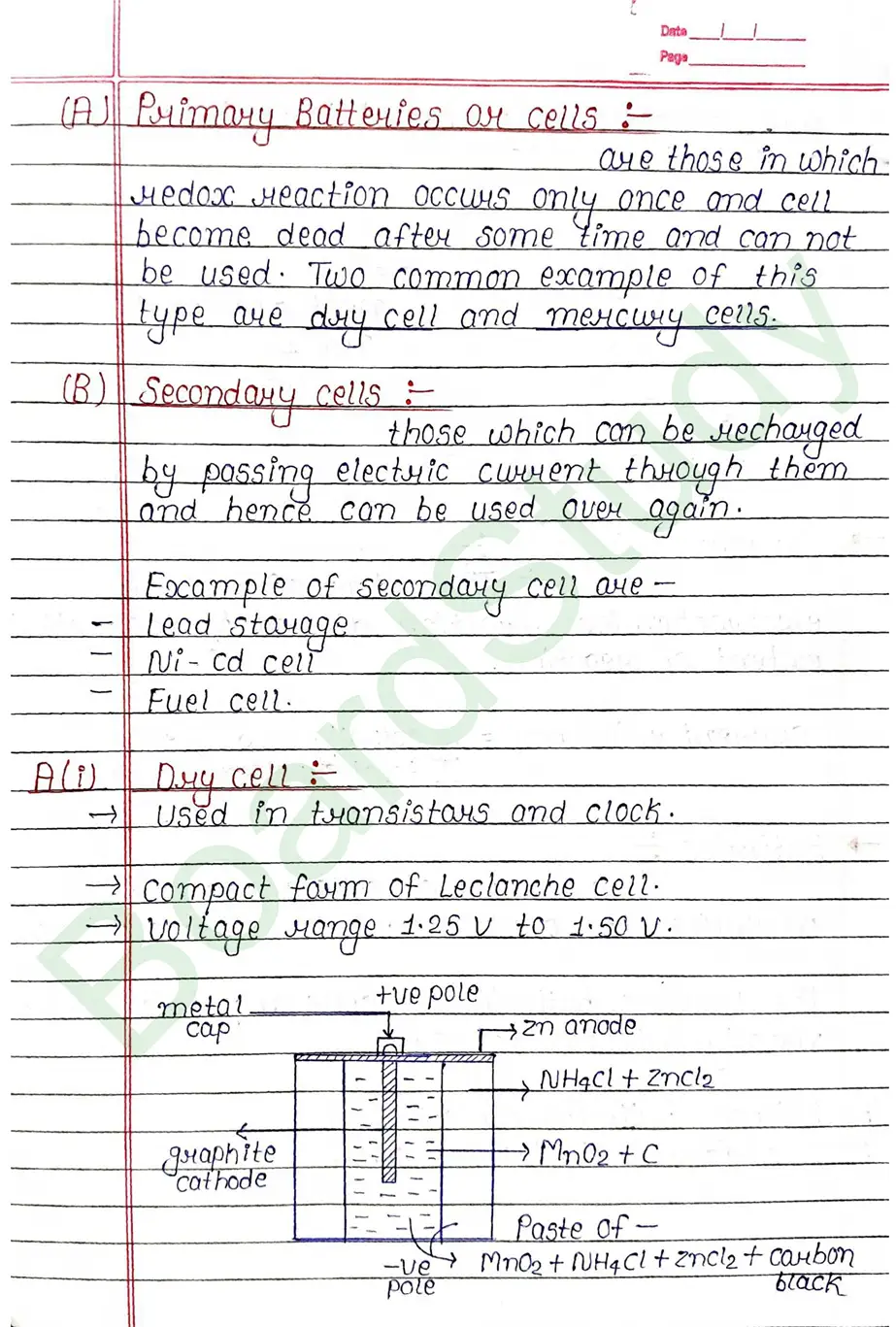
Conductance Conductance is the ability of an electrolytic solution to allow electric current to pass through it. eg: Metals graphite and certain minerals etc.
Electrolytes are further classified into –
- Strong Electrolyte : A strong electrolyte is a substance that completely dissociates into ions when dissolved in water, resulting in high electrical conductivity.
eg : HCl, HNO3, H2SO4 [strong acid] etc. - Weak Electrolyte : A weak electrolyte does not fully break into ions, so it conducts electricity weakly in solution. eg.
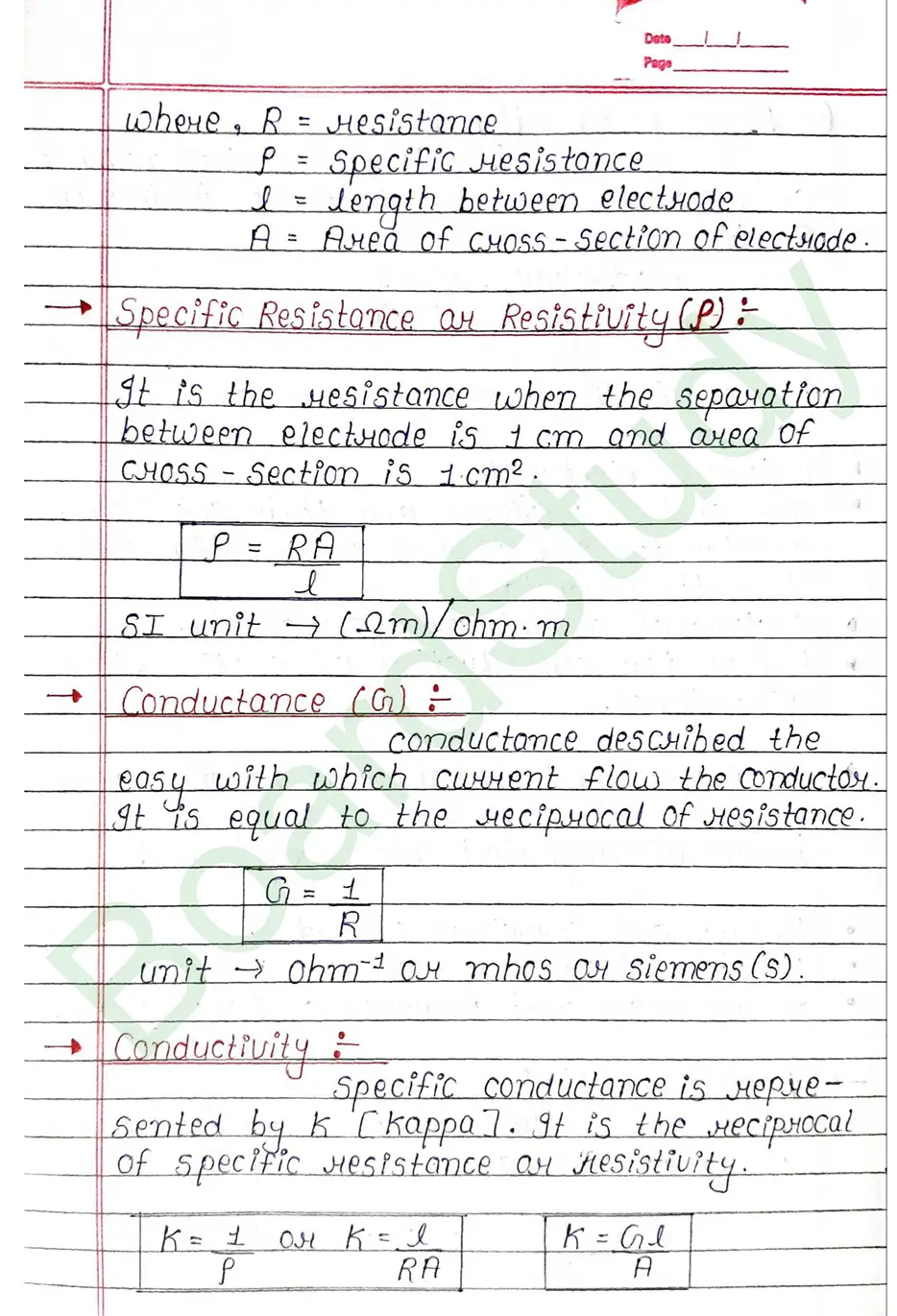
Conductance (G) conductance described the easy with which current flow the conductor. It is equal to the reciprocal of resistance.
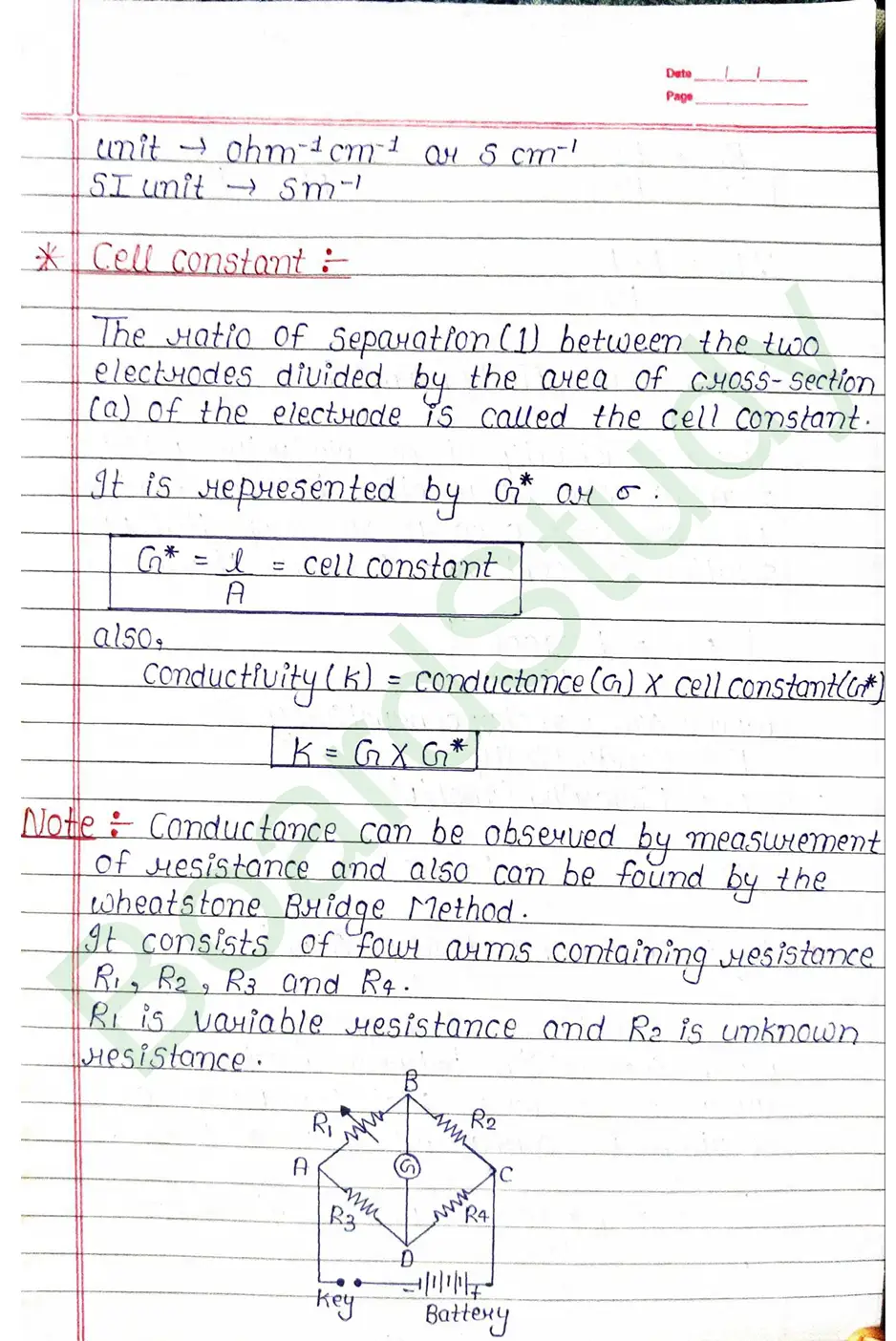
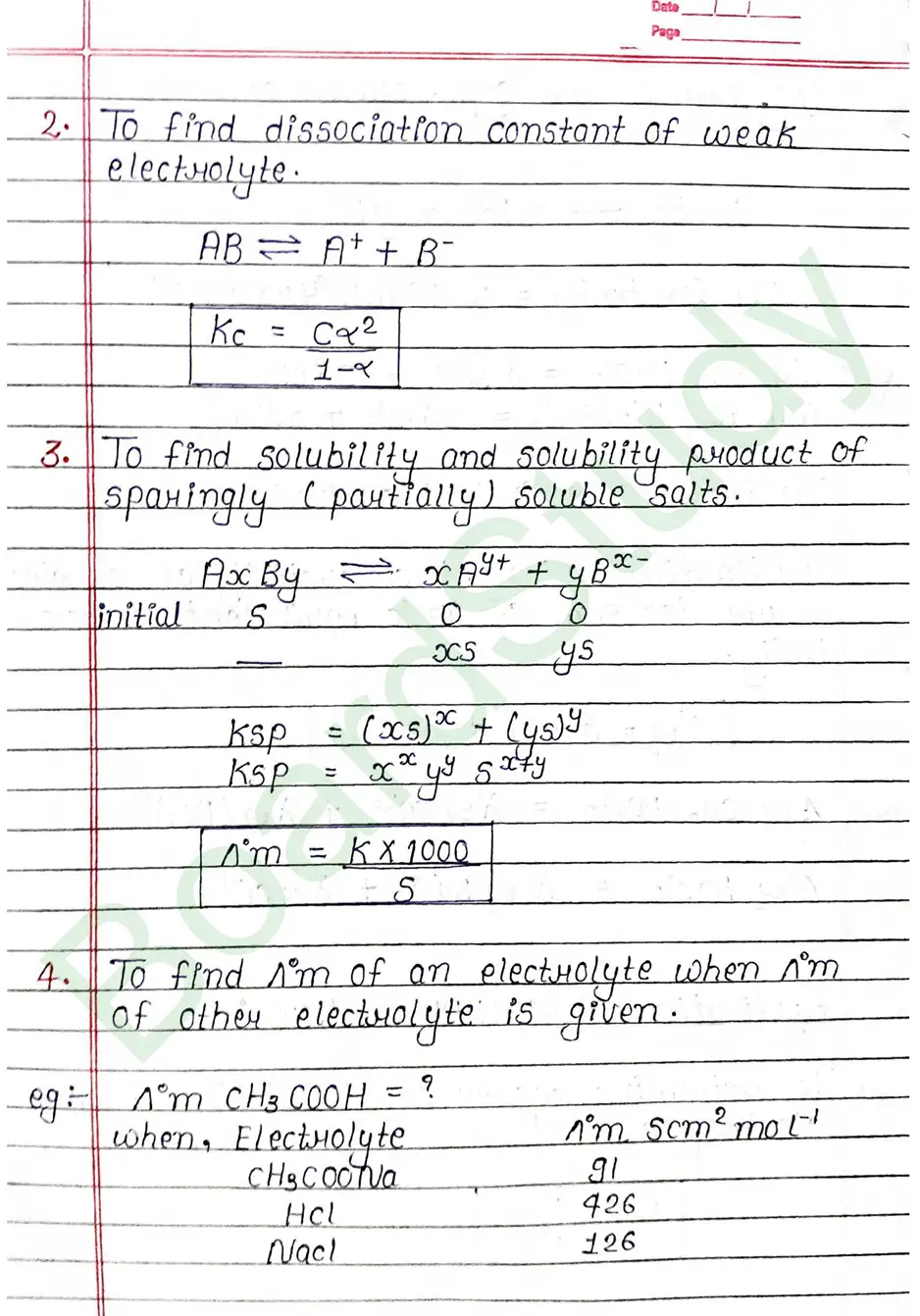
Cell constant: The ratio of Separation (1) between the two electrodes divided by the area of cross-section (a) of the electrode is called the cell constant.
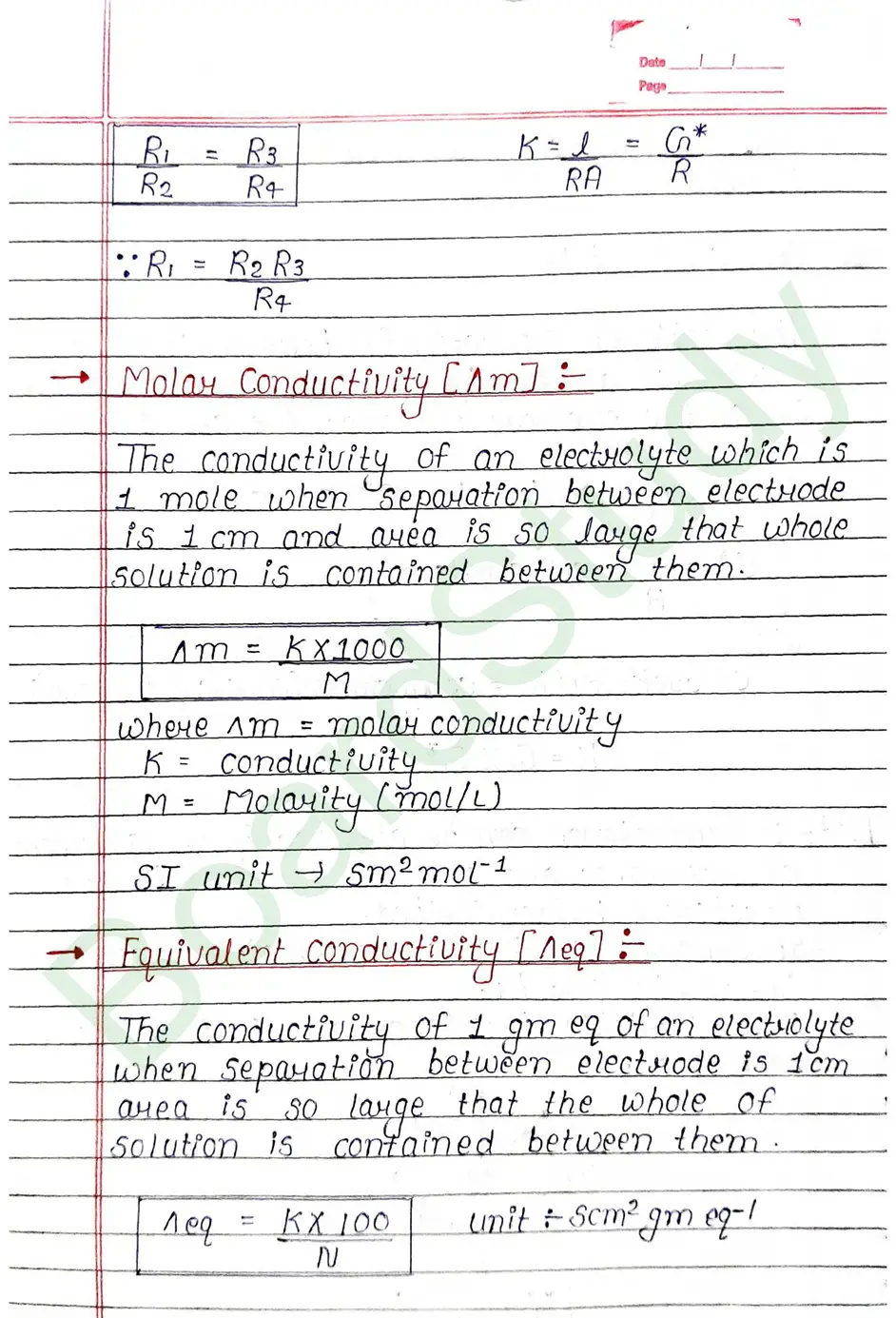
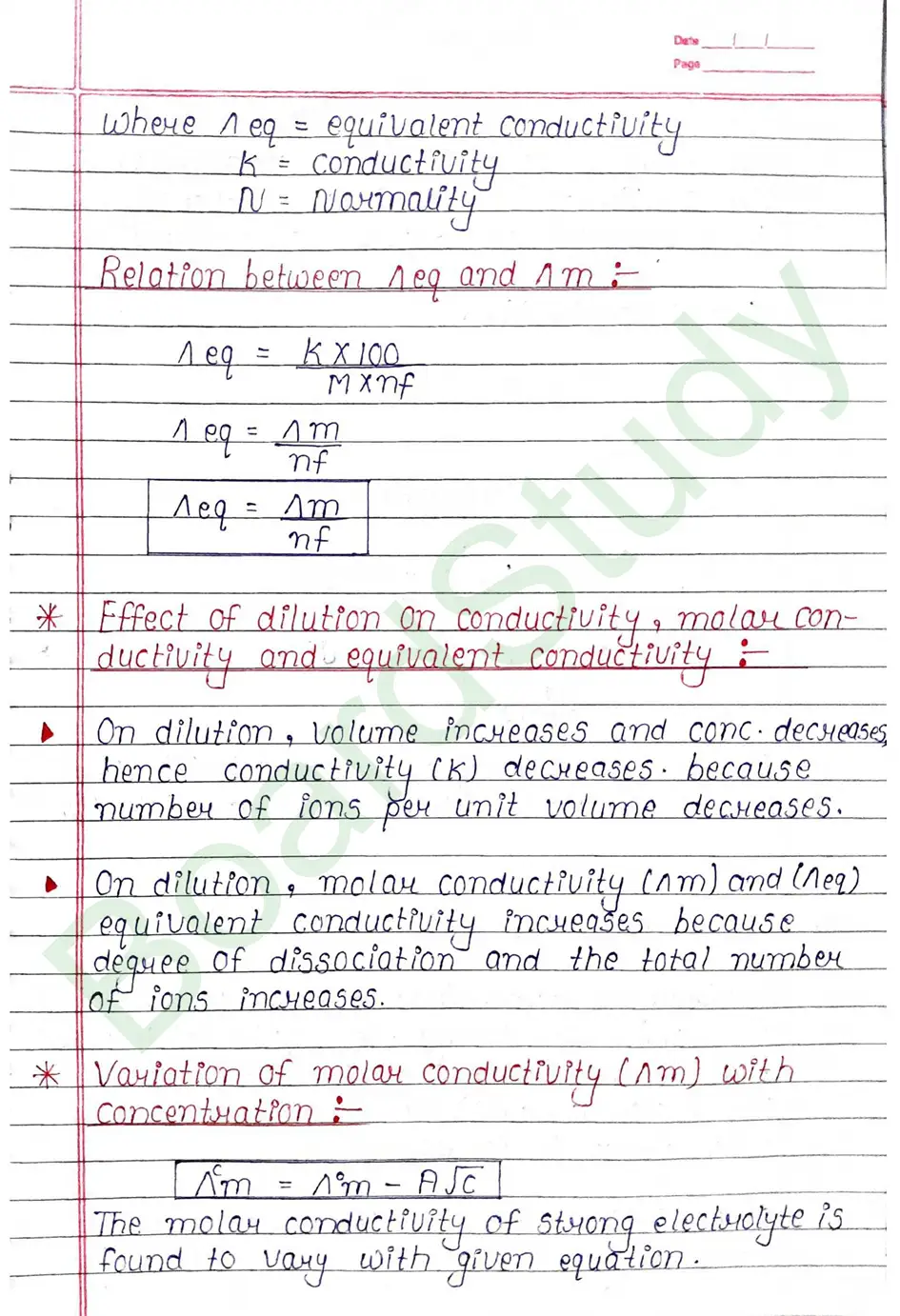
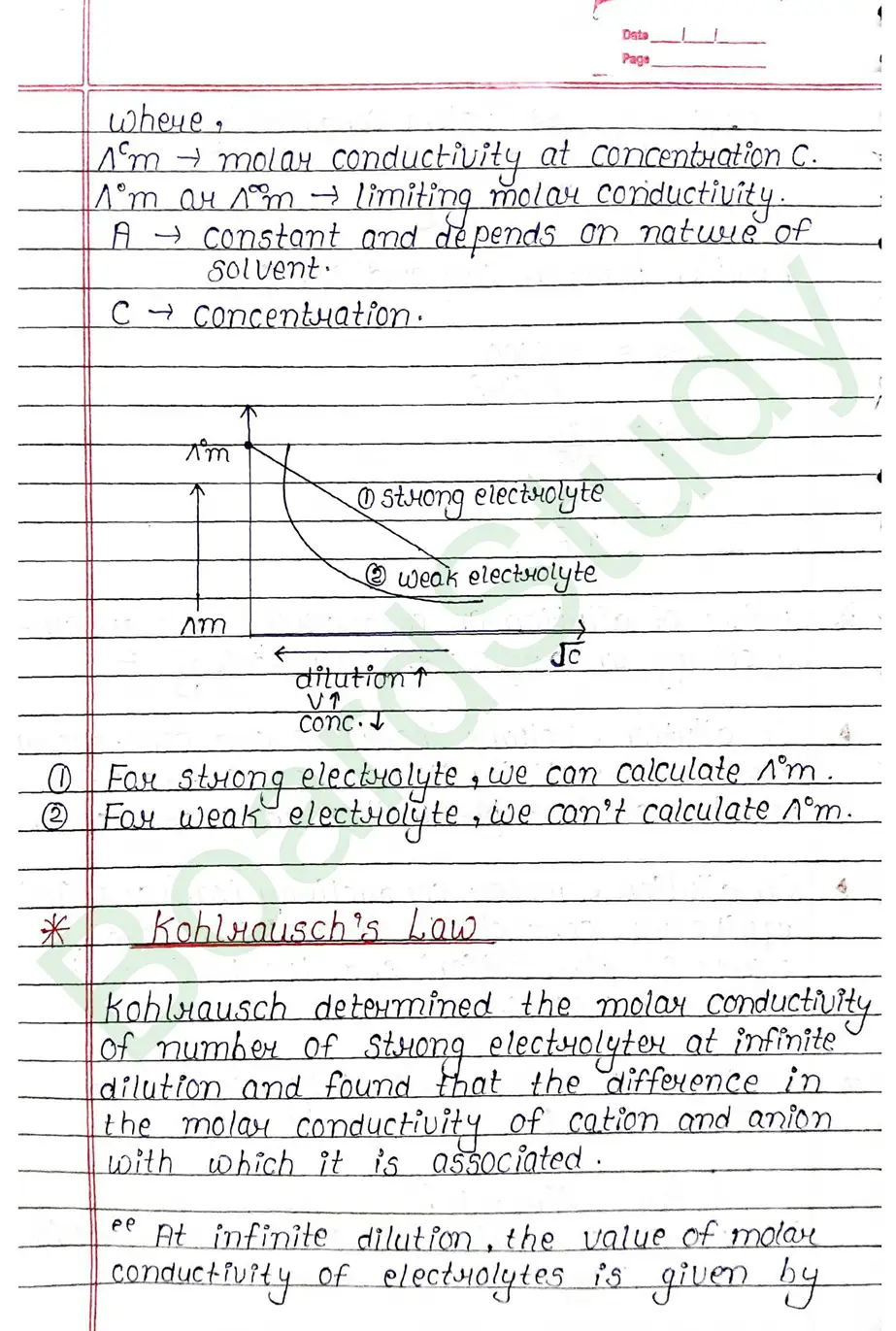
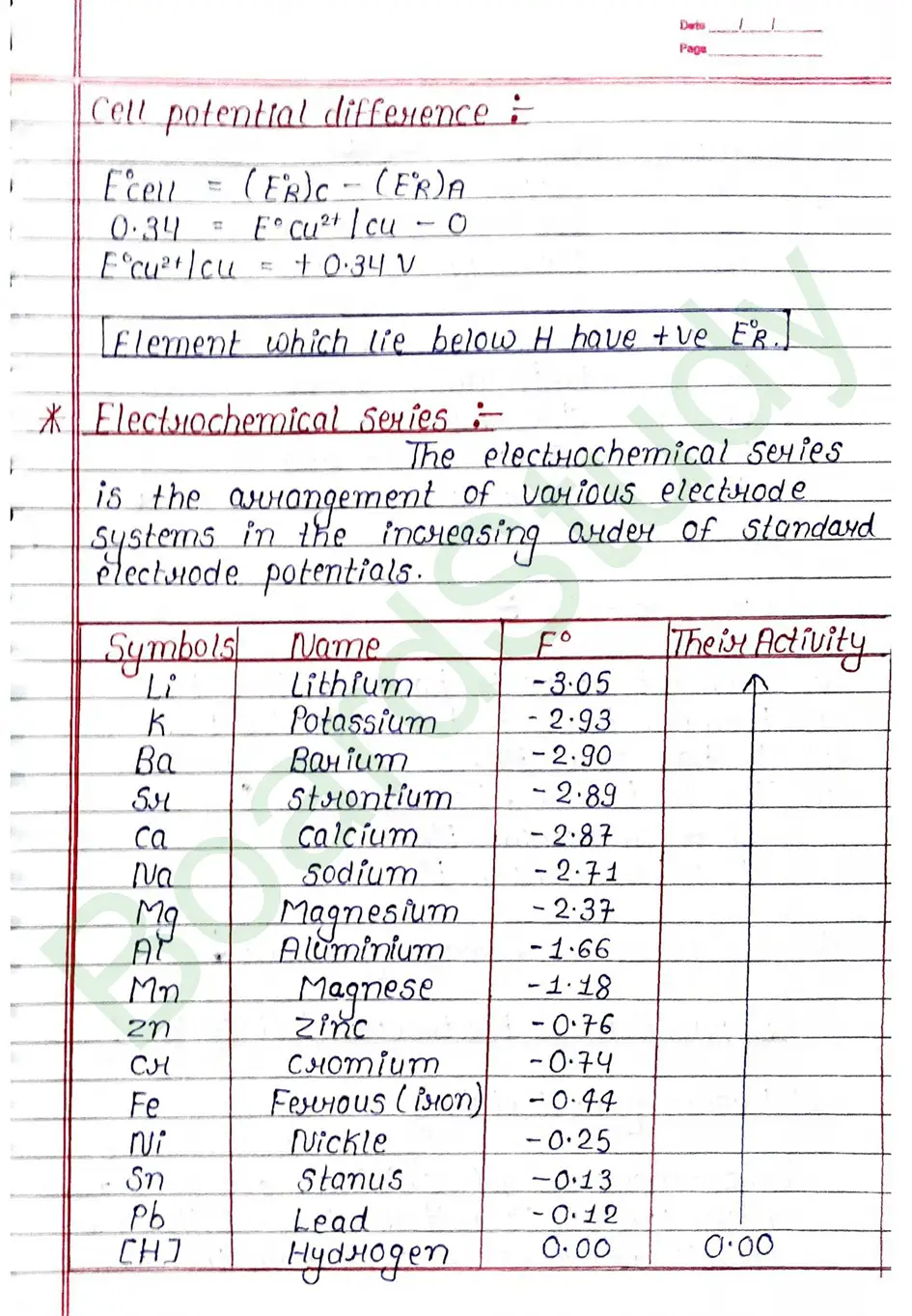
Kohlrausch’s Law Kohlrausch determined the molar conductivity of number of strong electrolyter at infinite dilution and found that the difference in the molar conductivity of cation and anion. with which it is associated.
At infinite dilution, the value of molar conductivity of electrolytes is given by the sum of the contribution of these ions (cations & anions).
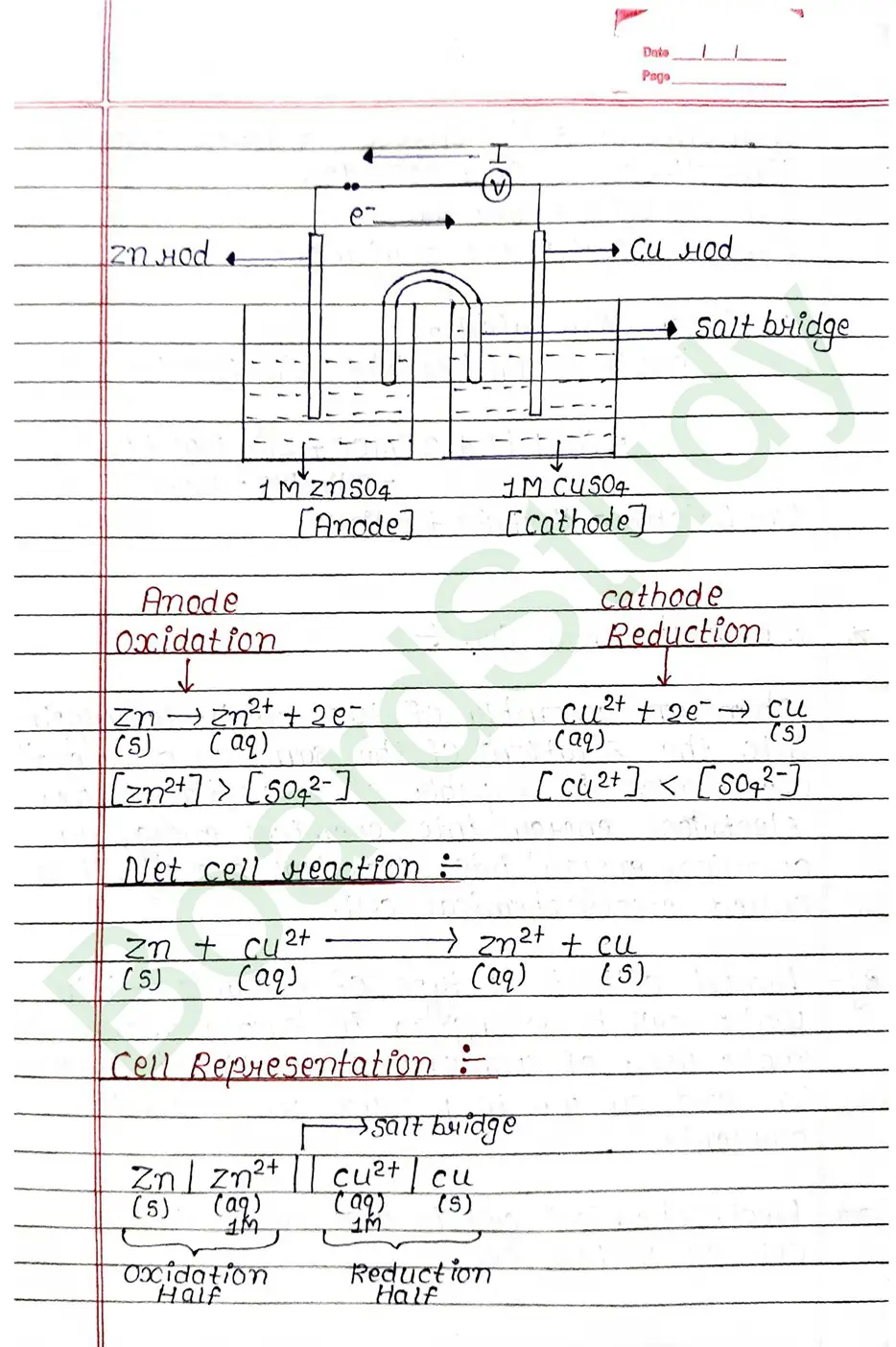
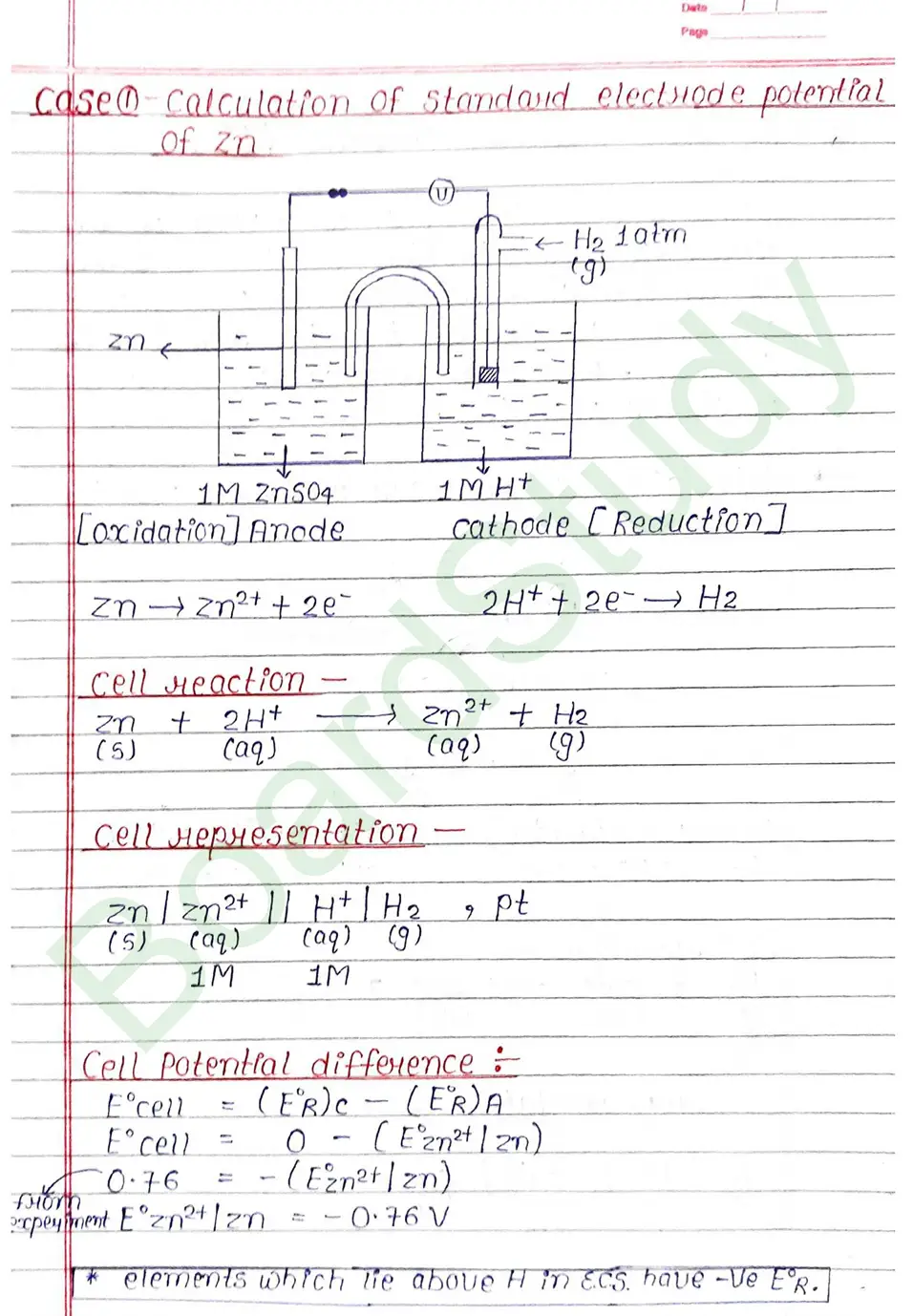
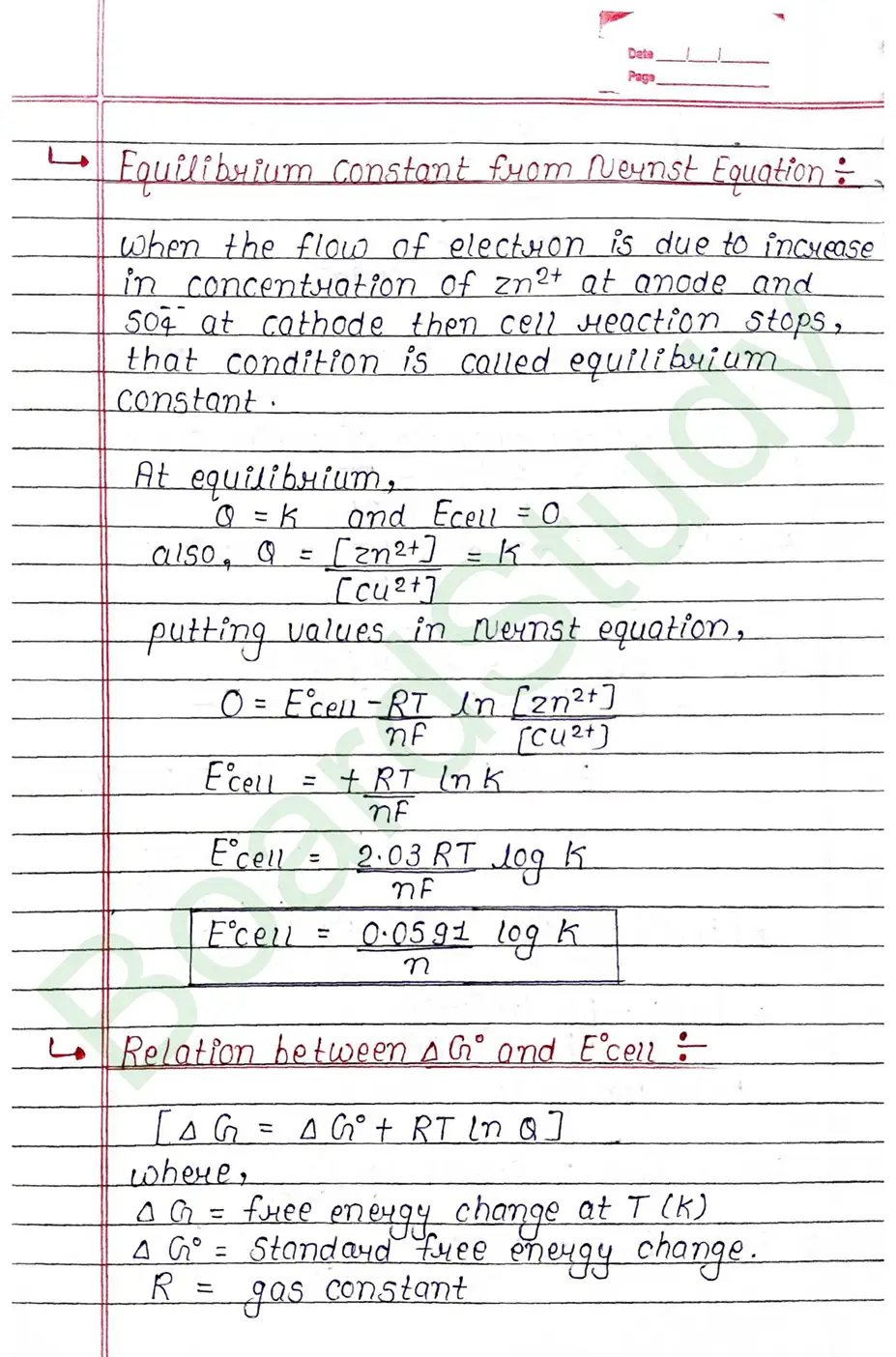
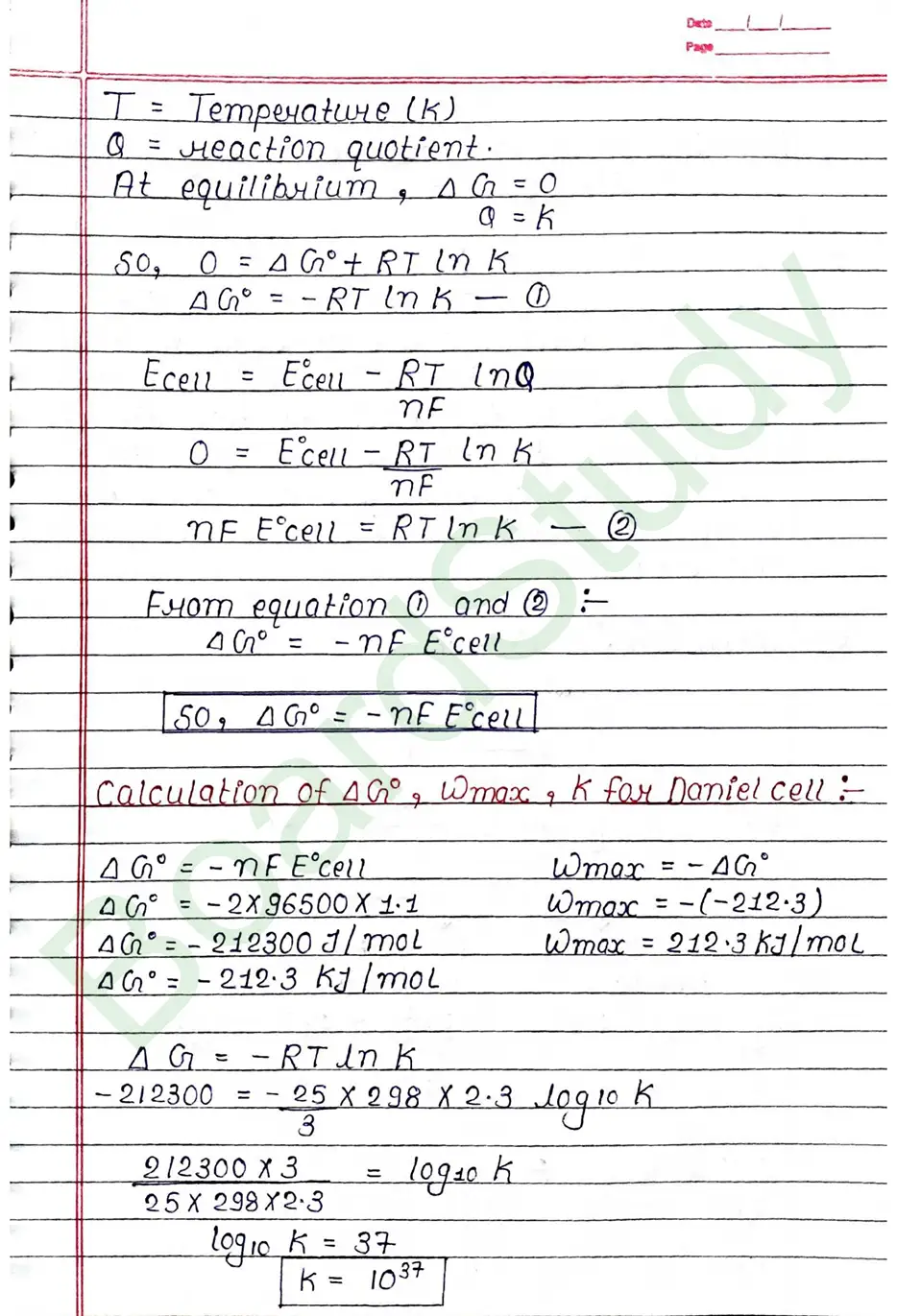
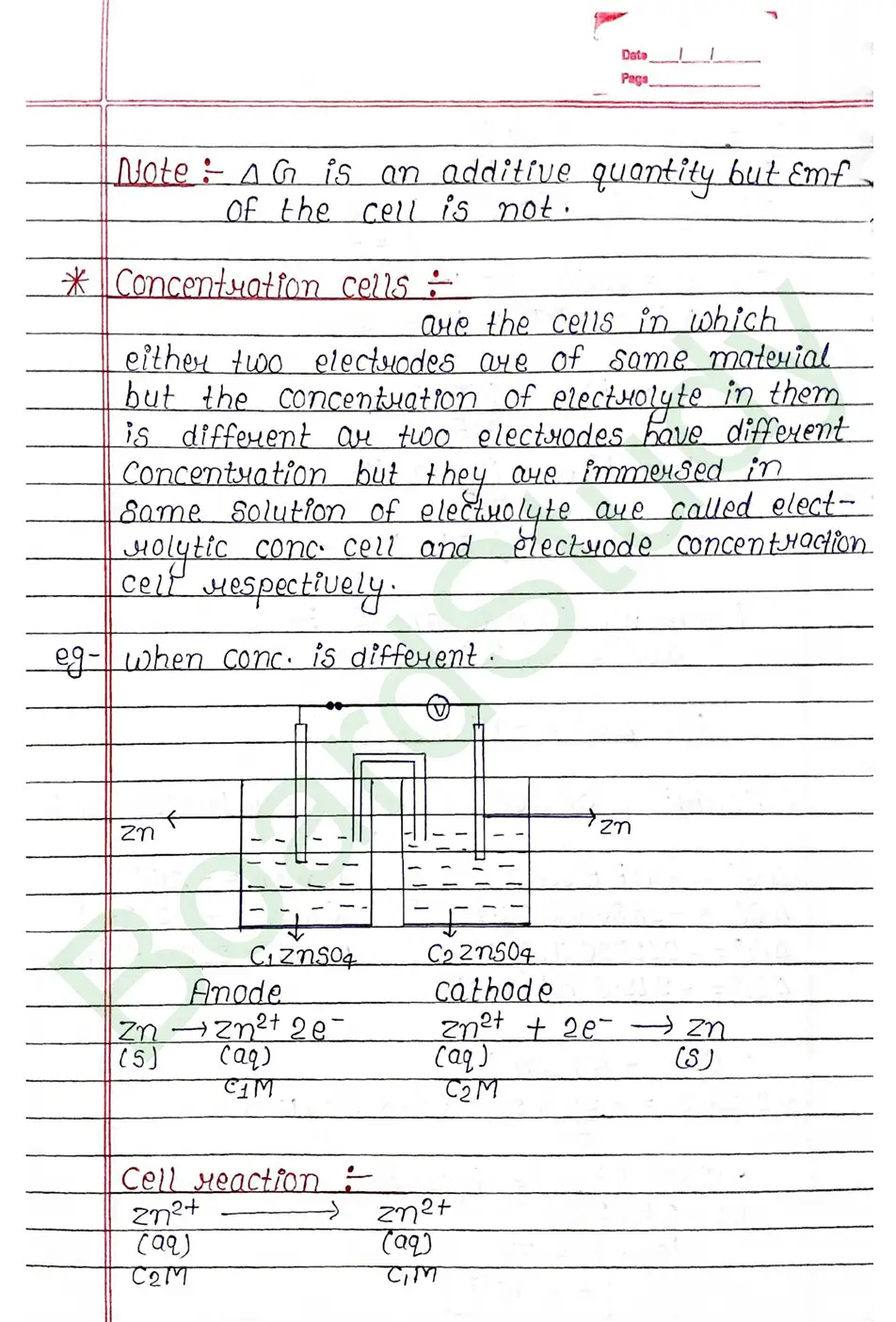
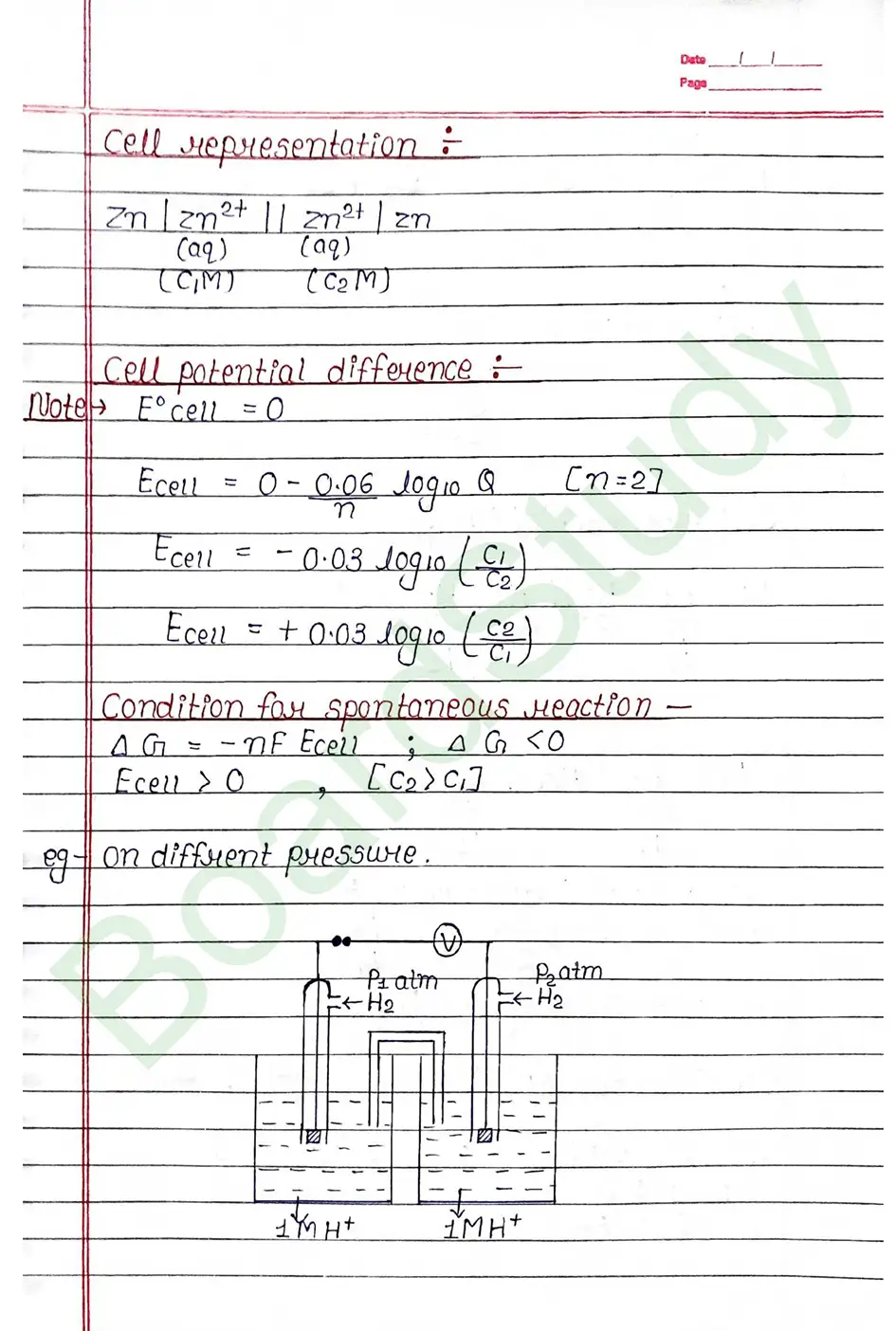
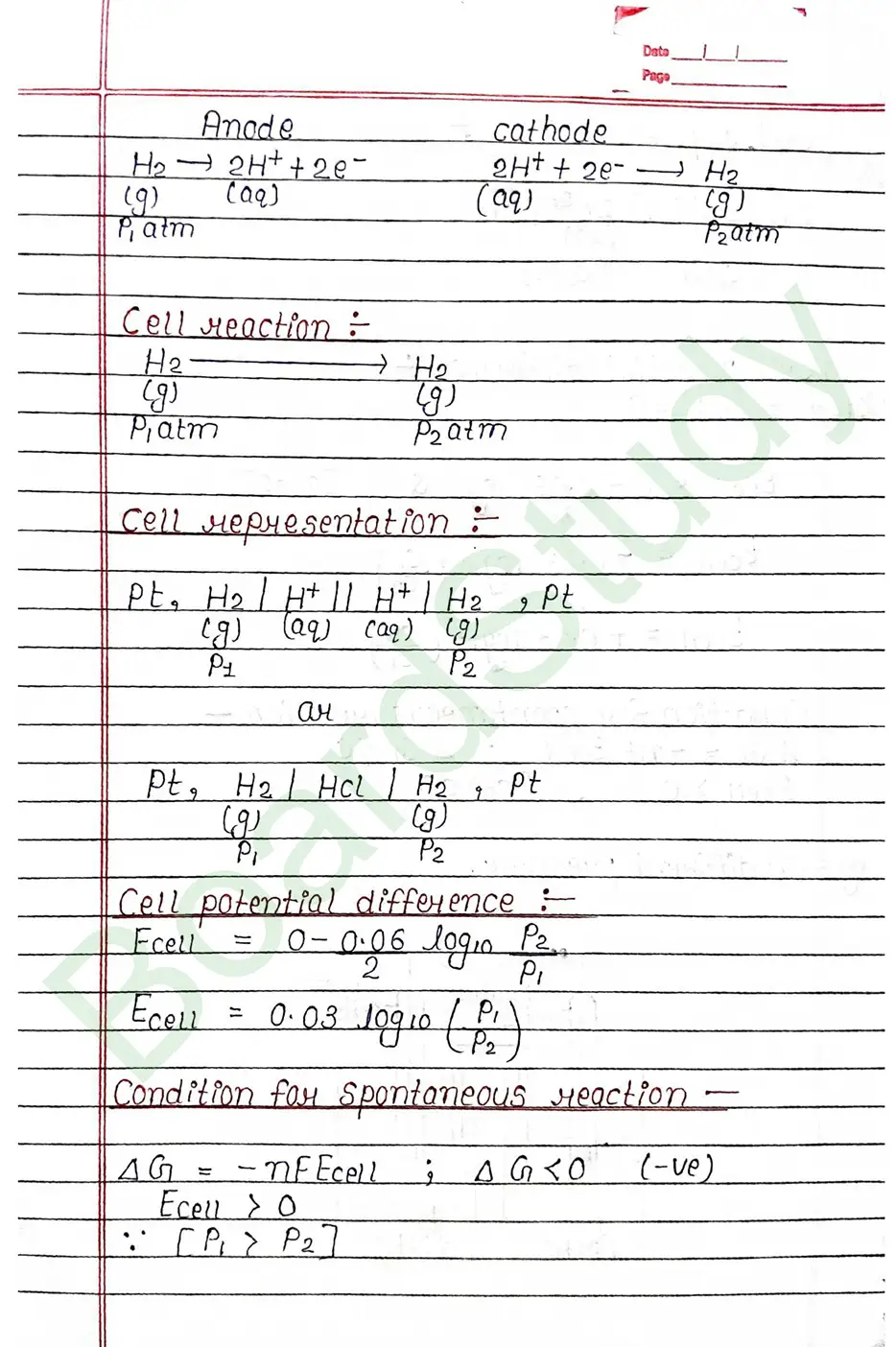
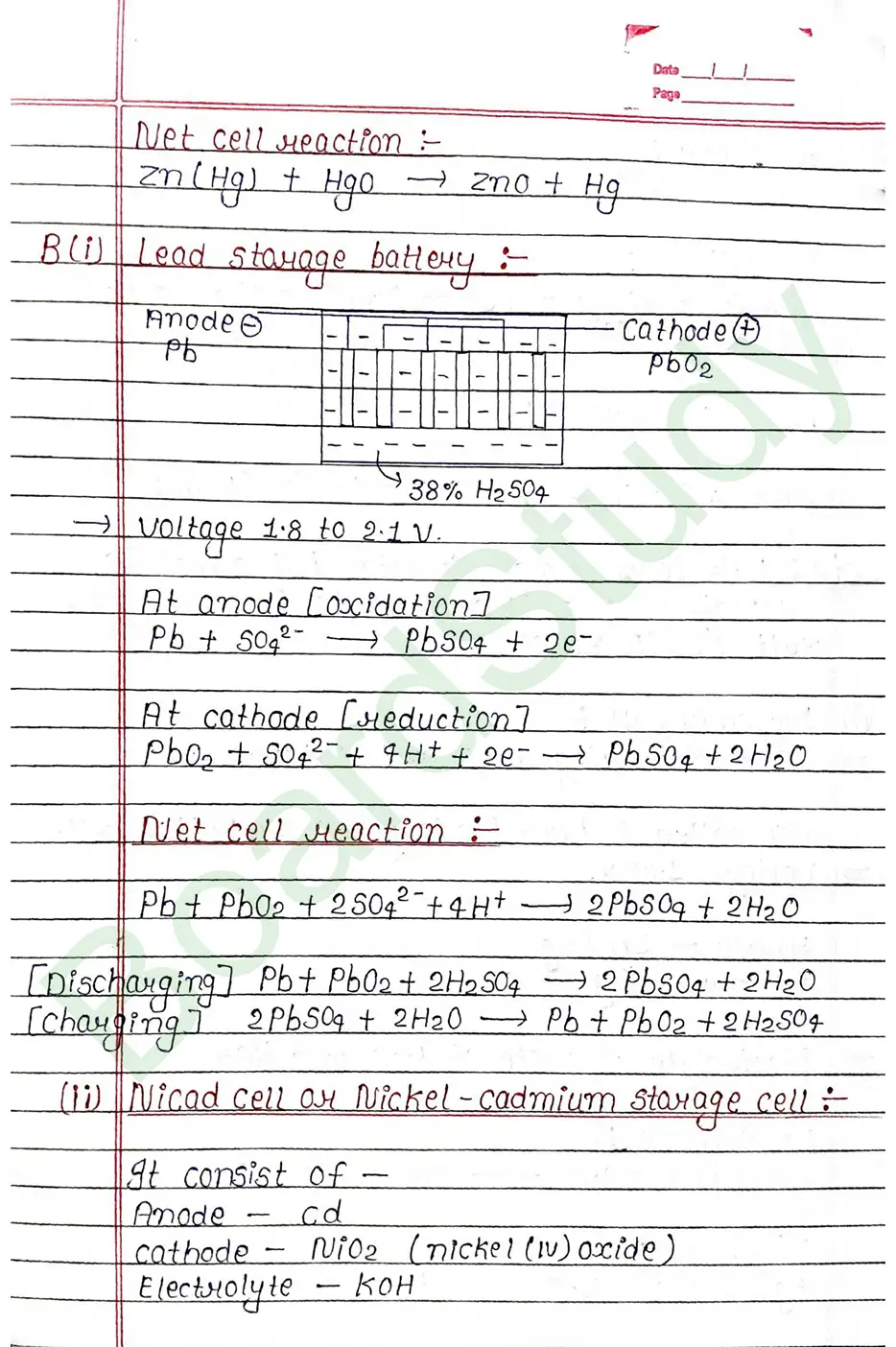
Electrochemical cell When an assembly of two electrodes dipping into the solution of the same or different electrolytes is capable of converting either electrical energy into chemical energy or chemical energy into electrical energy, it is called electrochemical cell.
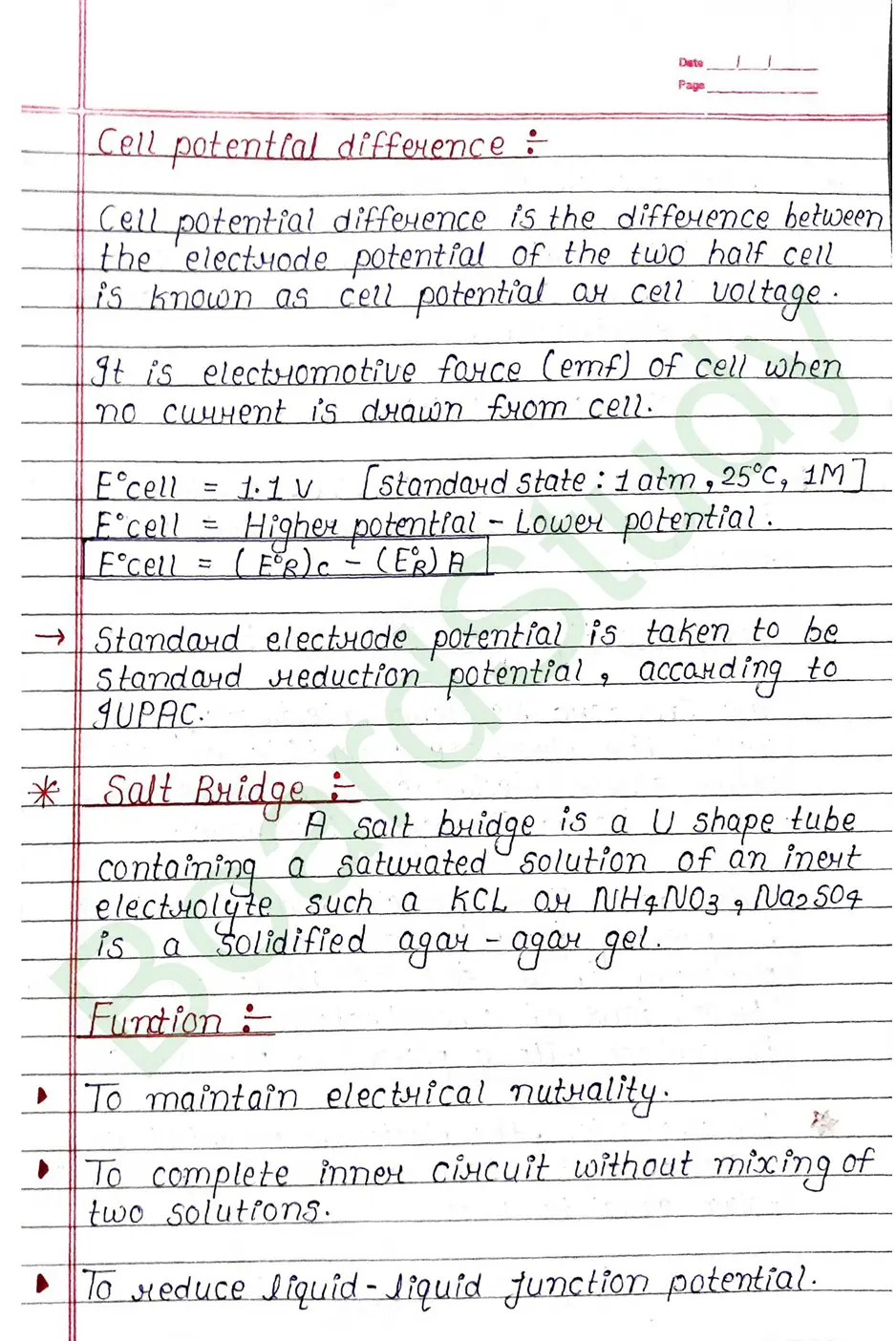
Salt Bridge
Salt Bridge: A salt bridge is a U shape tube containing a saturated solution of an inert electrolyte such a KCL ar NH4NO3, Na2SO4 is a solidified agar-agar gel.
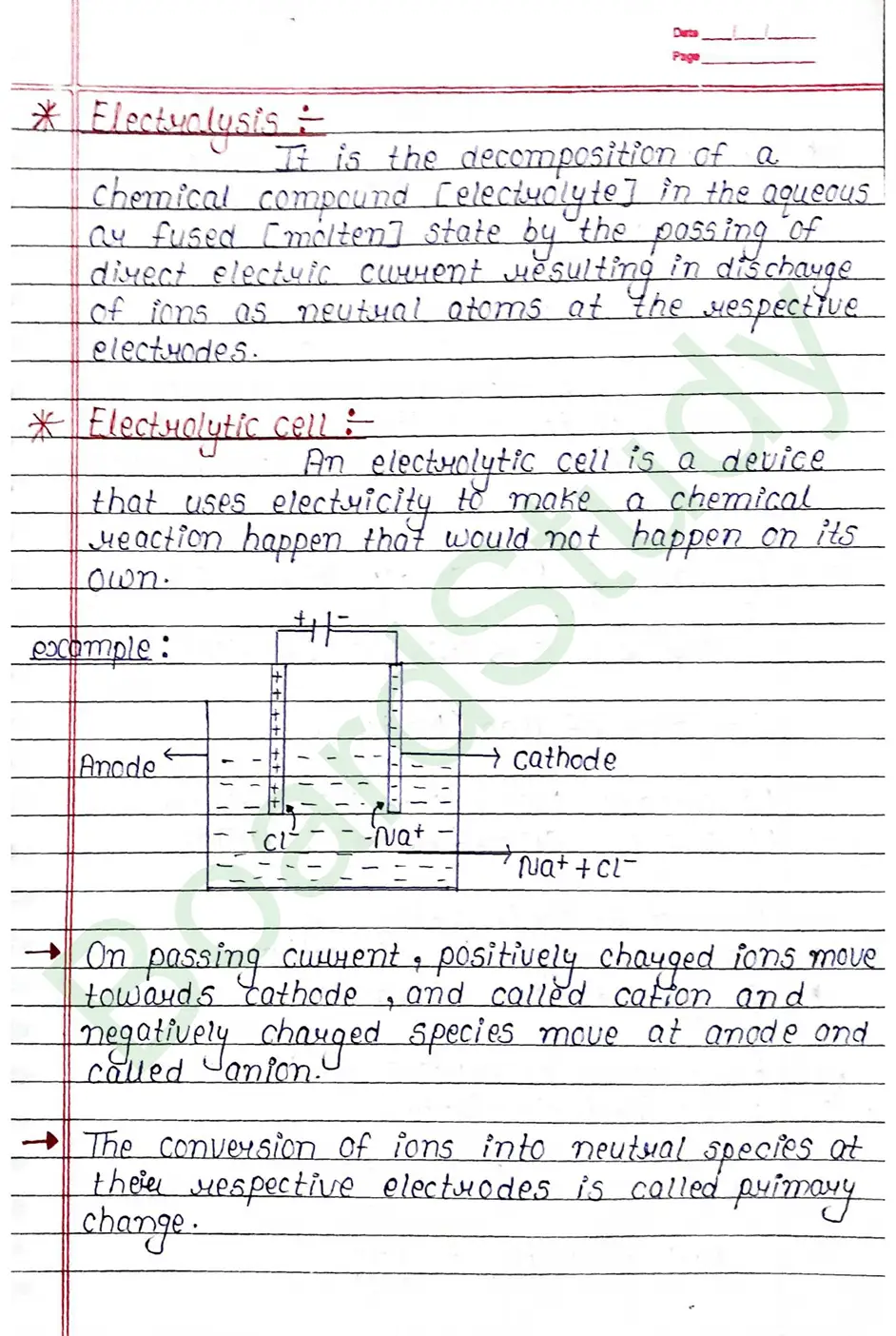
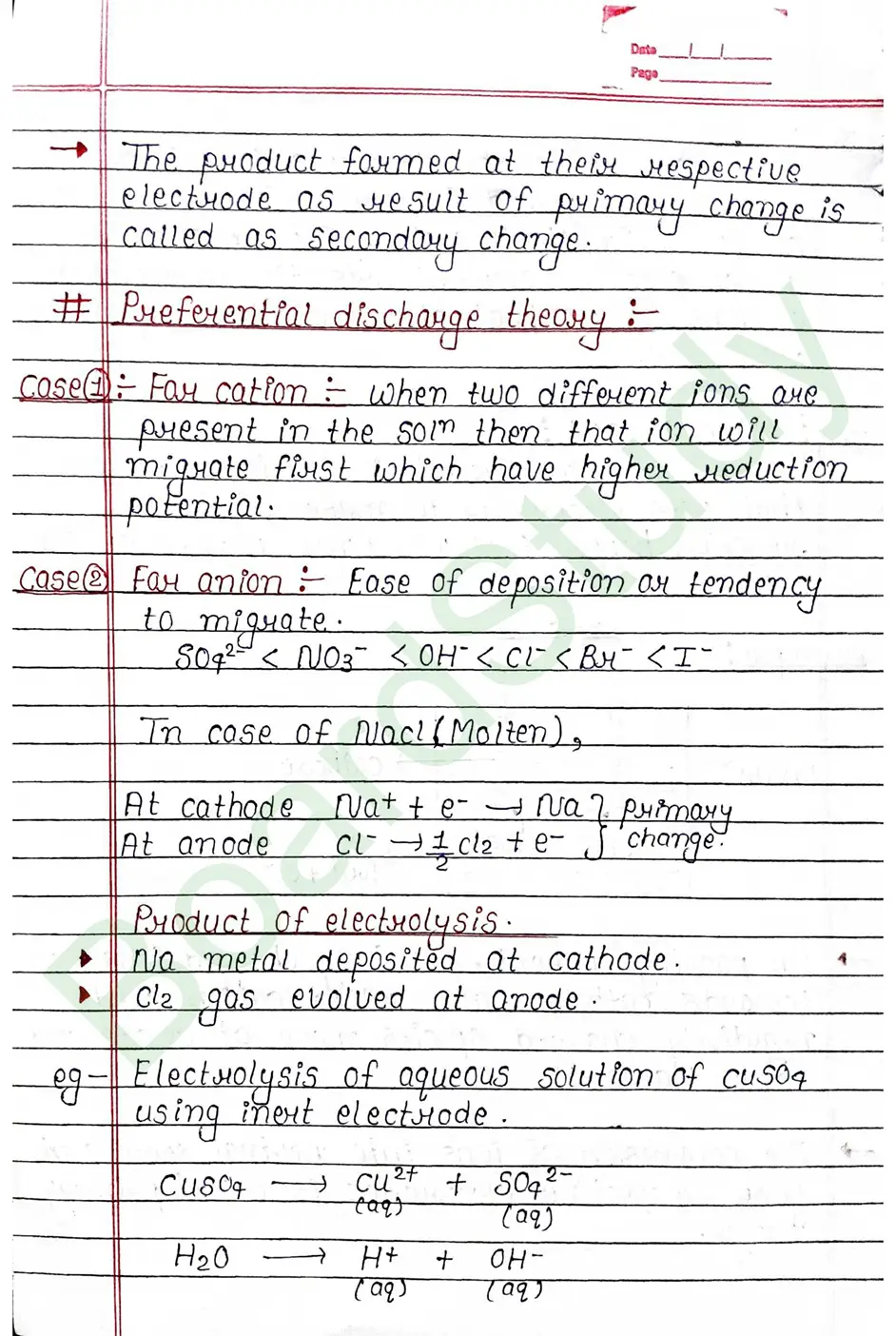
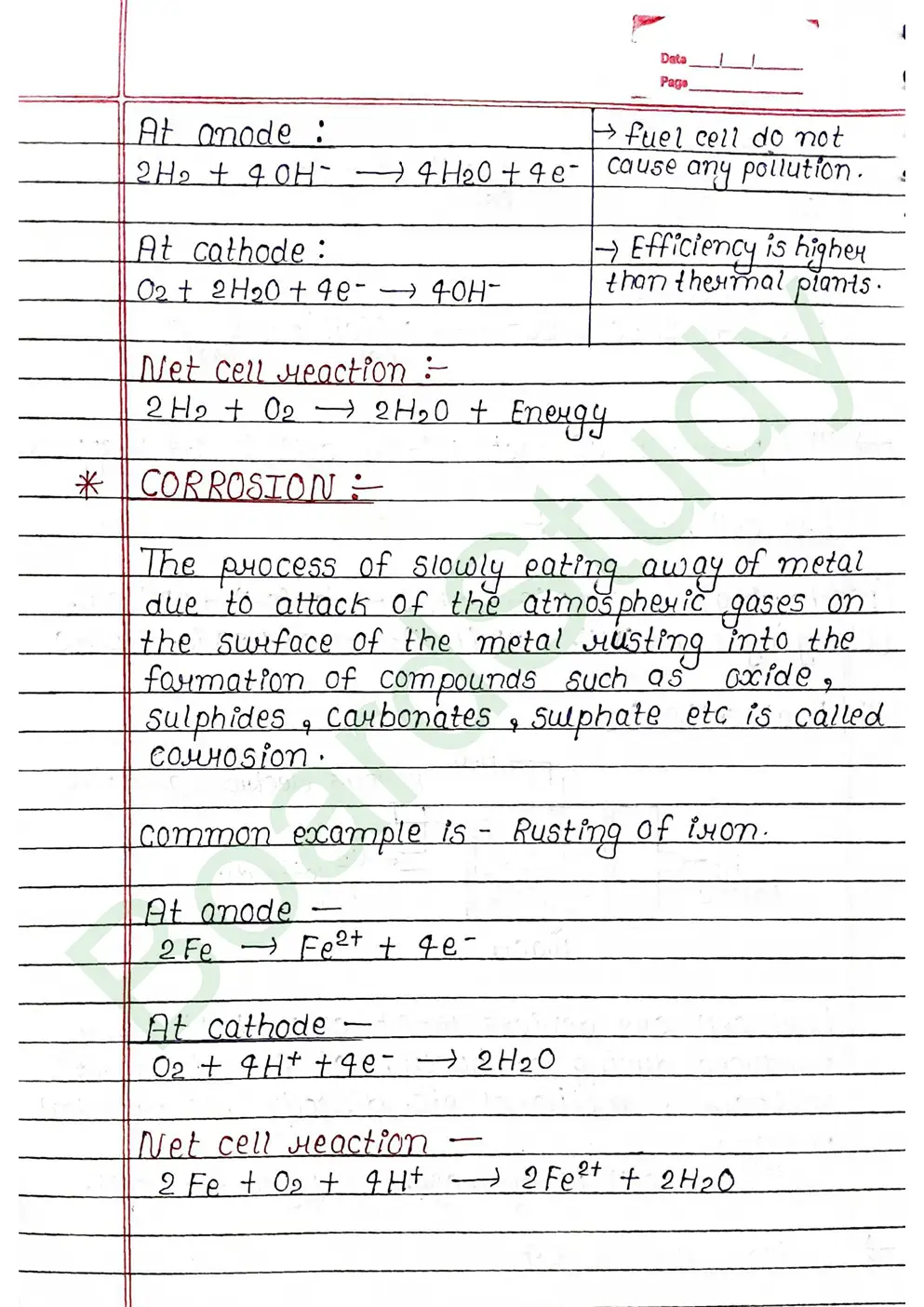
Electualysis It is the decomposition of a chemical compound (electrolyte] in the aqueous ay fused [molten] state by the possing of direct electric current resulting in discharge of ions as neutual atoms at the respective electrodes.
Electrolytic cell: An electrolytic cell is a device that uses electricity to make a chemical Meaction happen that would not happen on its own.
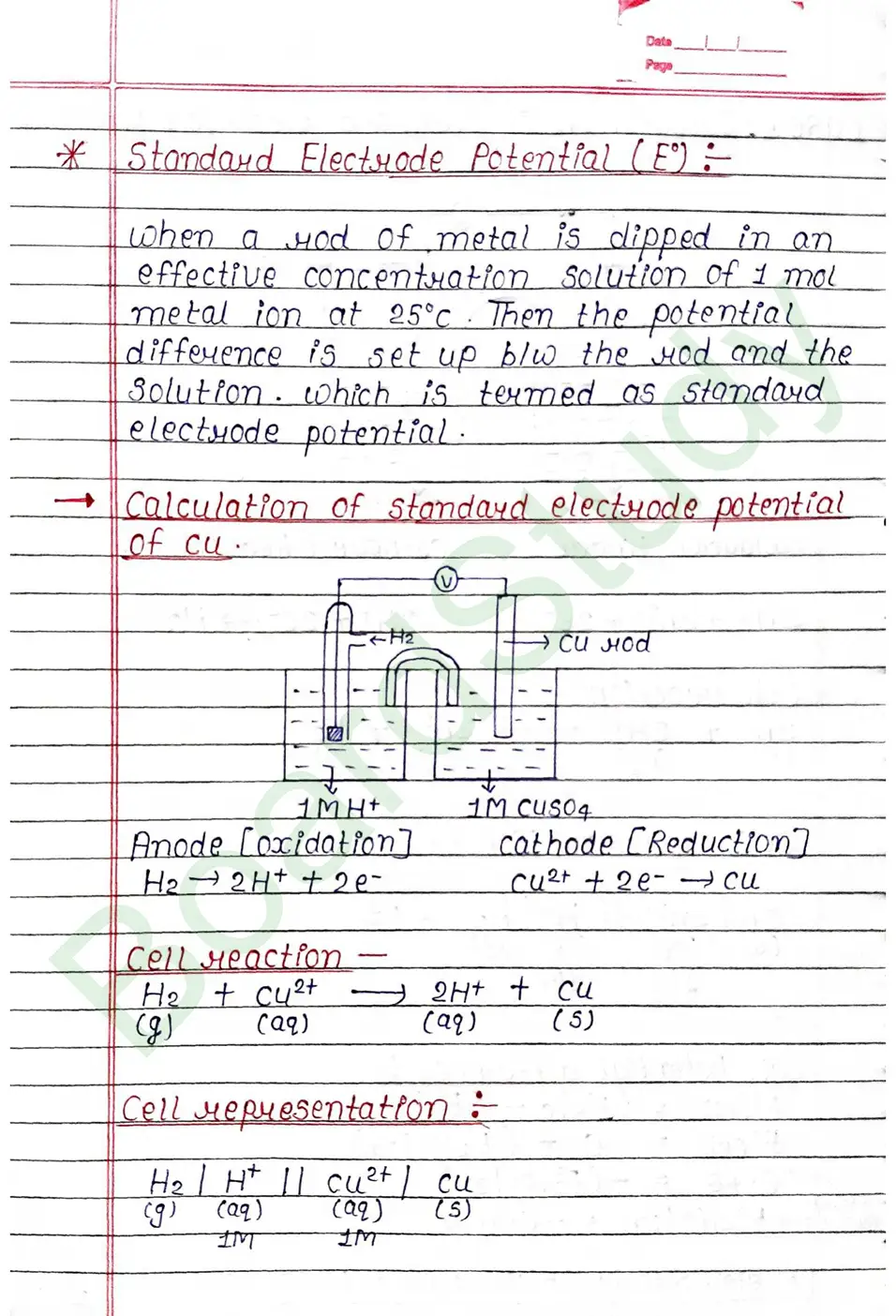
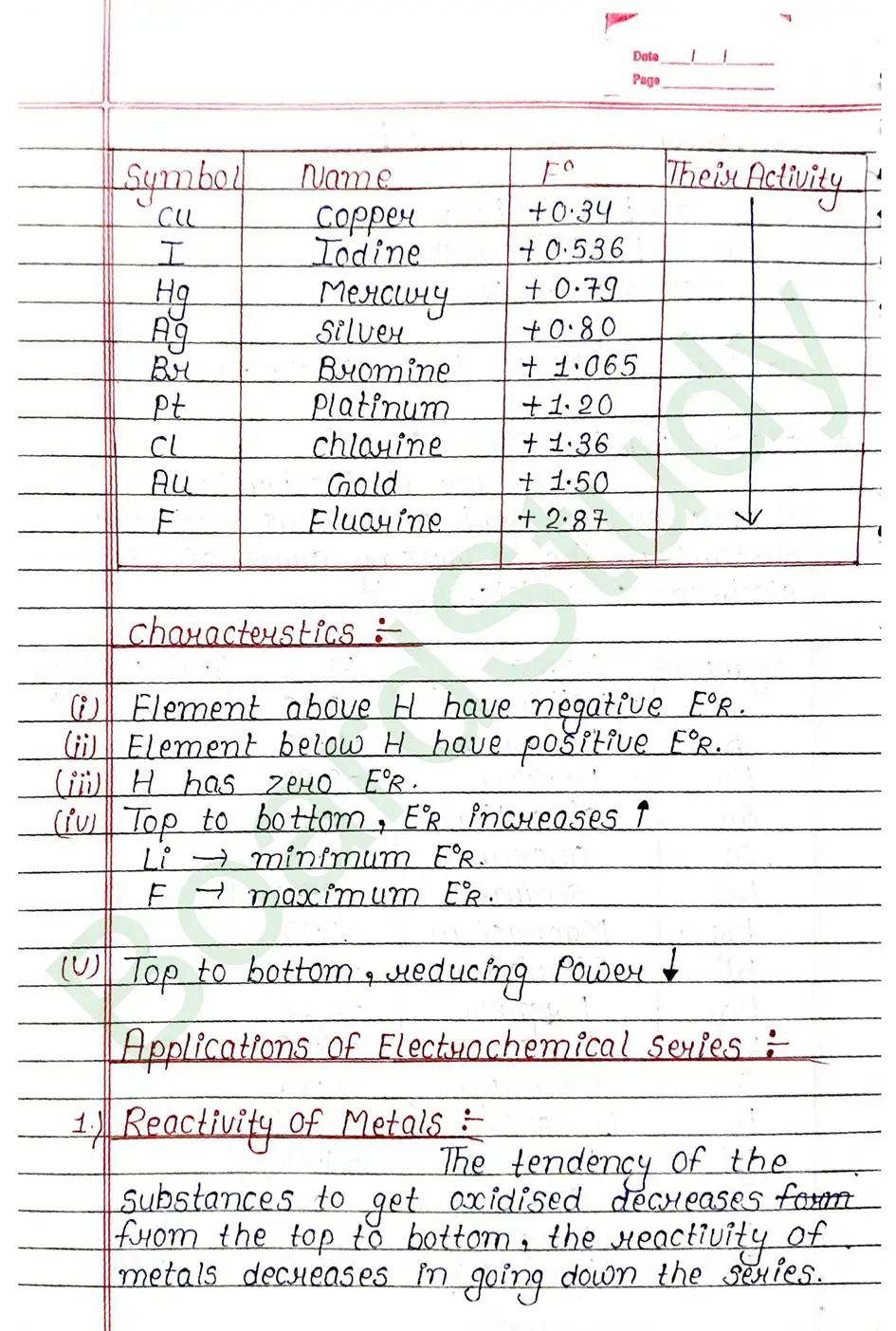
The standard electrode potential of an element is measured by constructing a cell with the Standard Hydrogen Electrode (SHE) as the other half-cell.
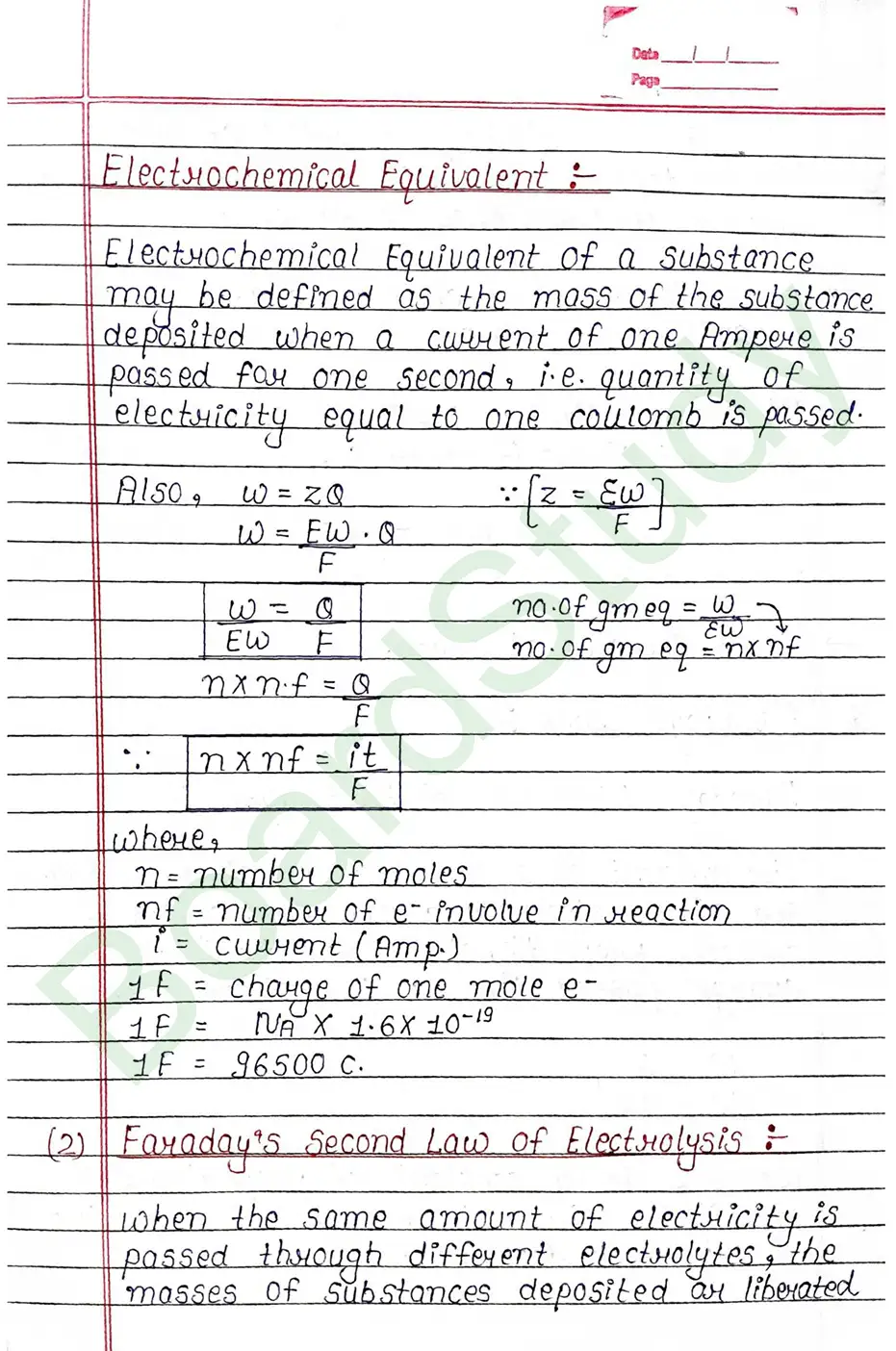
Faraday’s Laws
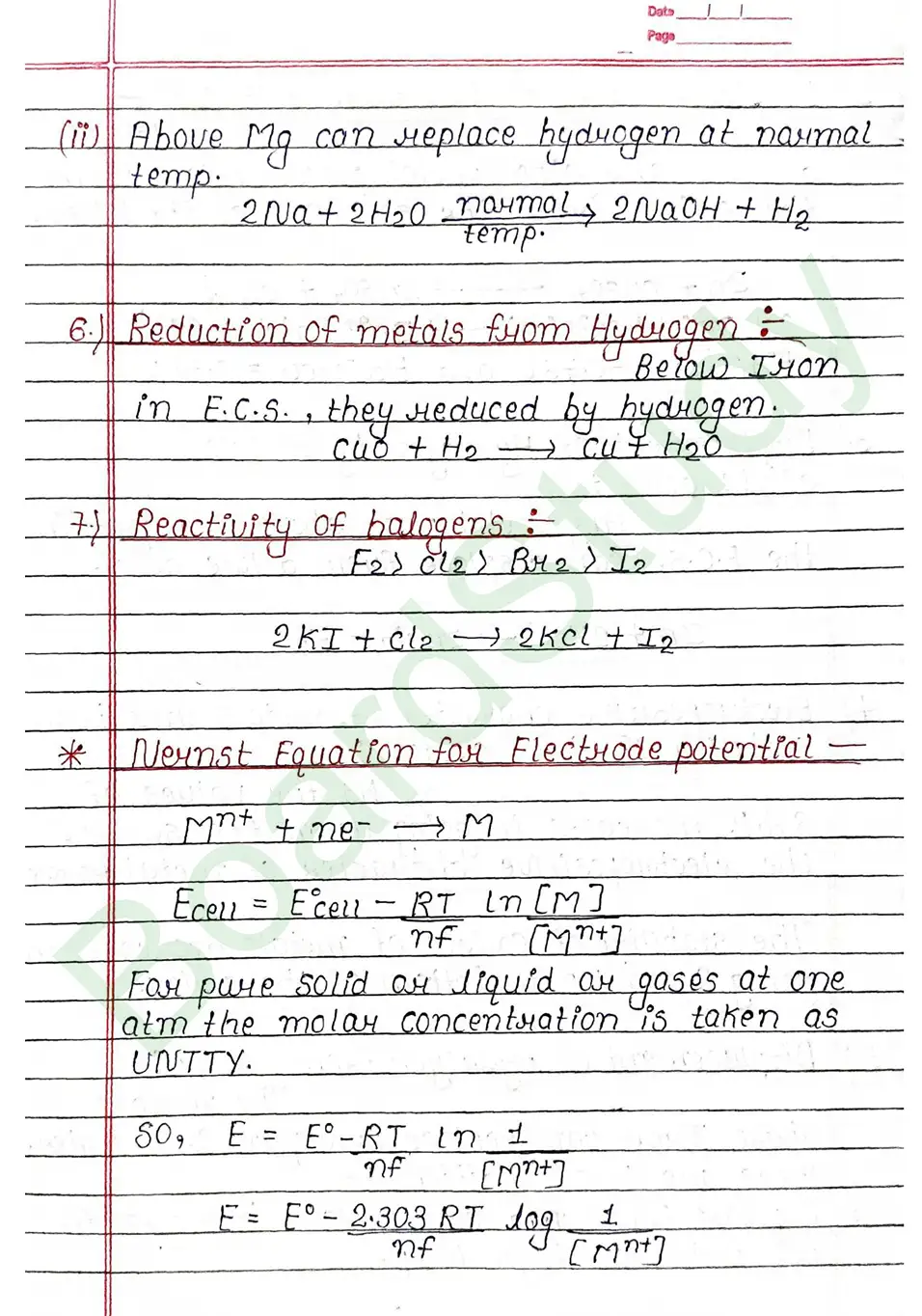
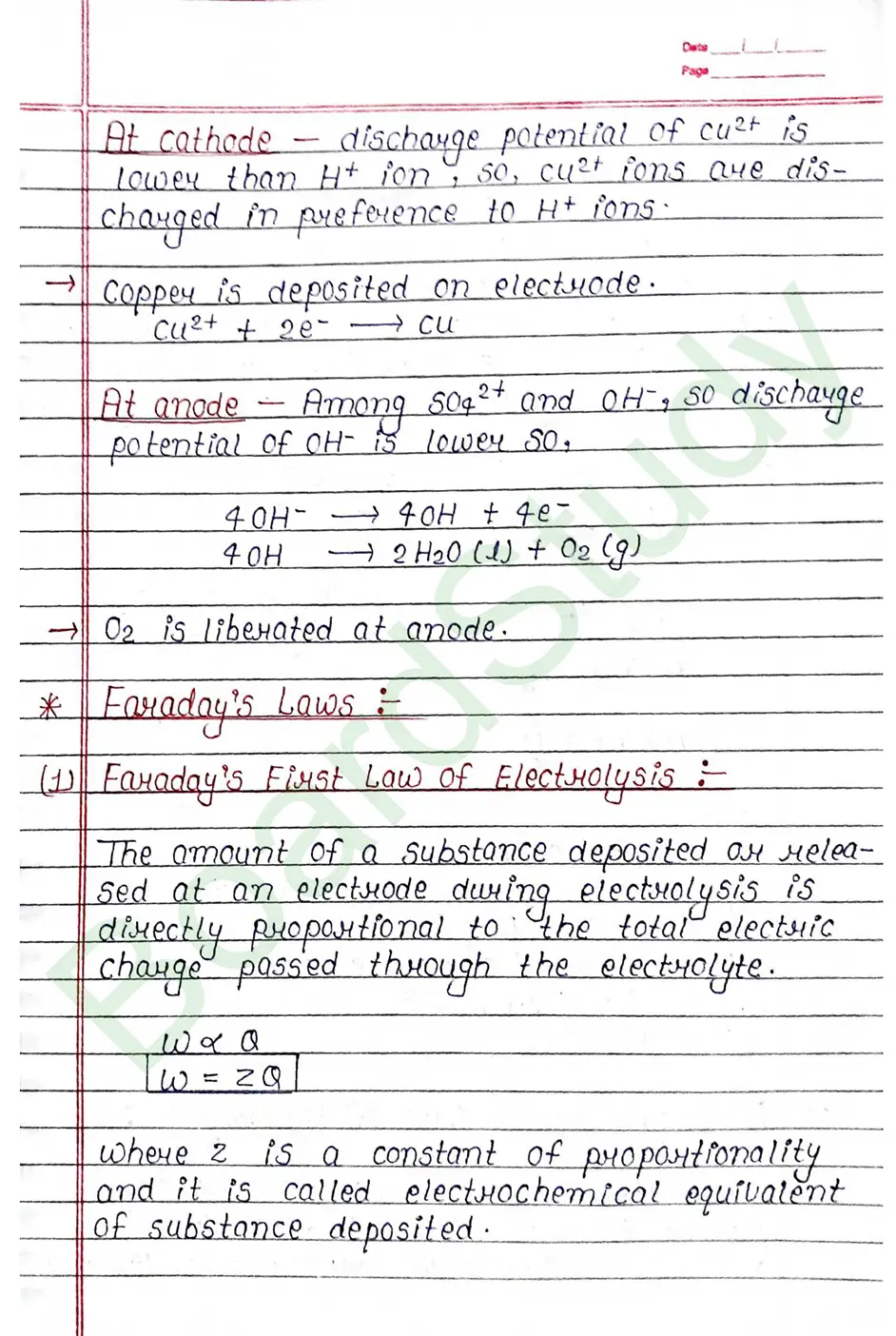
- Faraday’s First Law: The amount of a substance deposited or released at an electrode during electrolysis is directly proportional to the total electric charge passed through the electrolyte.
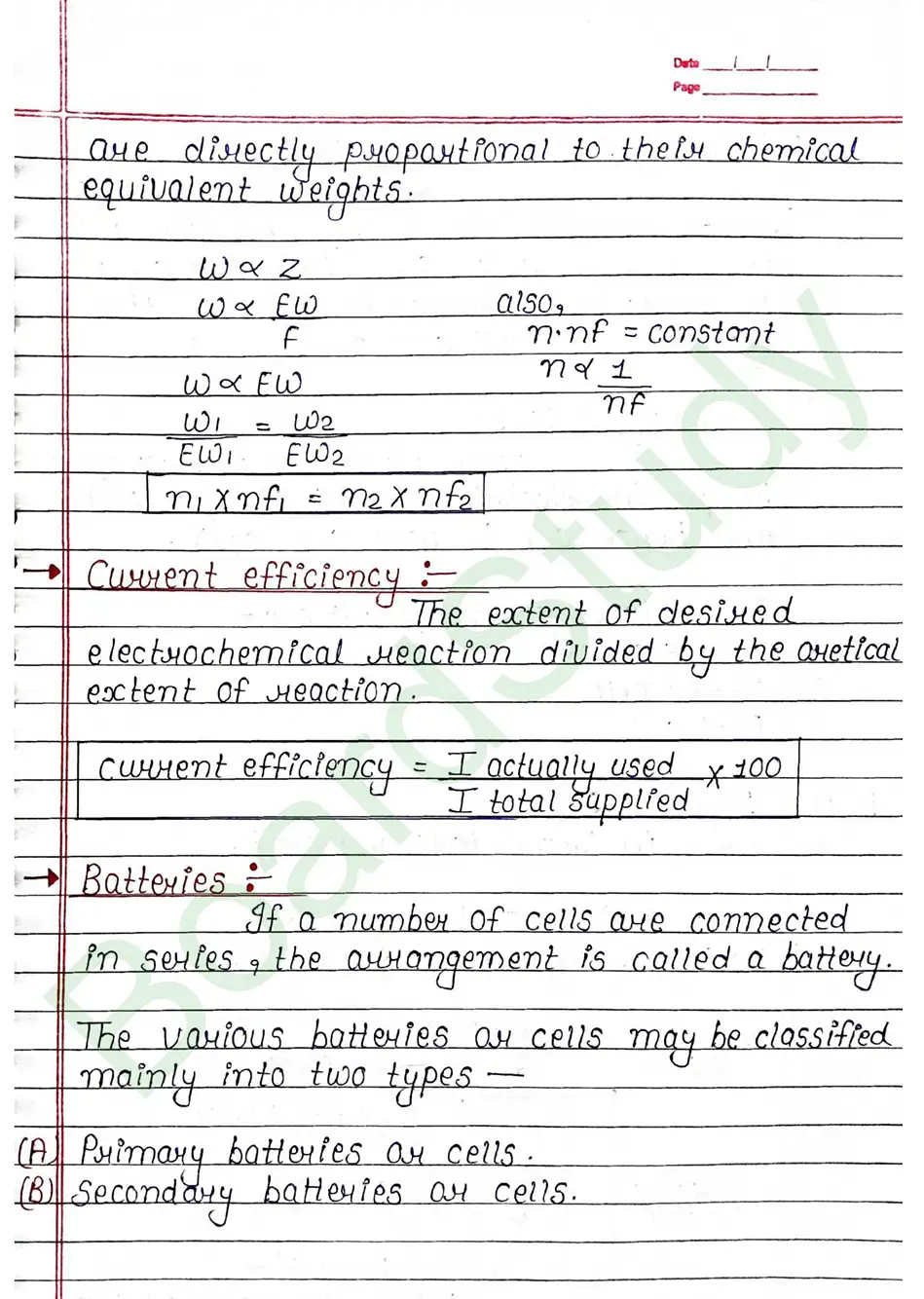
- Faraday’s Second Law: When the same amount of electricity is passed through different electrolytes, the masses of substances deposited or liberated are directly proportional to their chemical equivalent weights .
Features of Notes
- Students can use Electrochemistry notes for last minute revision.
- In the last few days of exam students feel very stress due to pressure of exam. Notes will be very helpful for managing the stress in the last days of exam.
- All notes are totally free of cost and students can access notes anytime on our for totally free of cost.
- Electrochemistry Notes PDF are created very carefully so you can rely on this notes.
Summary
| Chapter | Electrochemistry |
| Chapter Number | 2 |
| Subject | Chemistry |
| Class | 12 |
| Medium | English |
FAQ
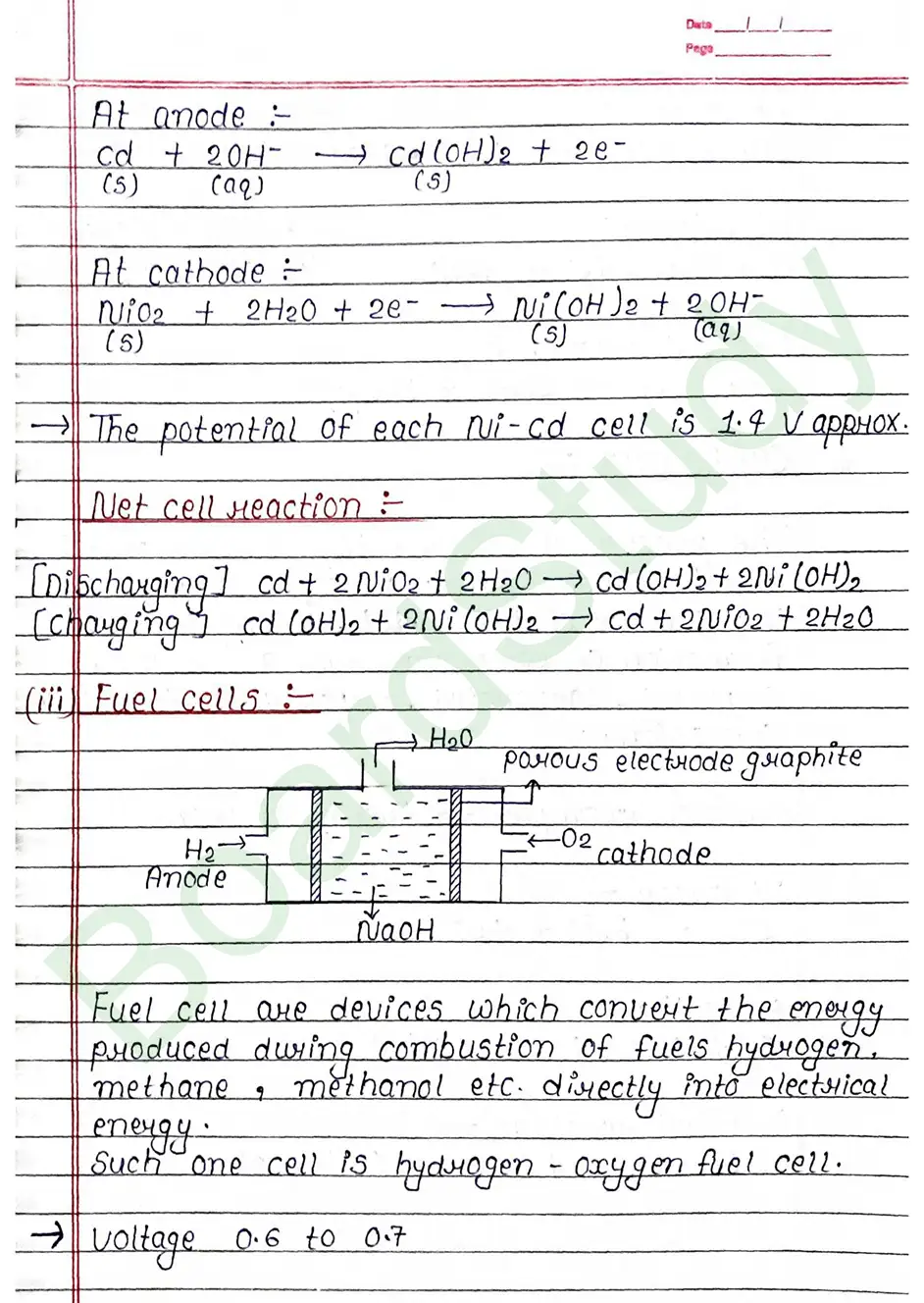
What is Fuel cell ?
Fuel cell are devices which convert the energy produced during combustion of fuels hydrogen, methane, methanol etc. directly into electrical energy.
What is Conductance (G) ?
Conductance (G) conductance described the easy with which current flow the conductor. It is equal to the reciprocal of resistance.
Are these notes sufficient for board exam?
Electrochemistry handwritten notes are created by topper’s and expert teacher keeping board exam in mind so you can score maximum in board exam.
Are Electrochemistry Handwritten notes according to NCERT latest syllabus?
Yes notes are created according to the NCERT latest syllabus.
How can i download Electrochemistry Notes PDF?
For downloading Electrochemistry Notes PDF click on Download PDF button.

Thanks for these. These are amazing-we can also see them on screen without those nonsense popups
🙌🙌
so helpful
very good