Here we have shared class 12 Chemistry Haloalkanes and Haloarenes Notes. The Haloalkanes and Haloarenes notes is a best resources for students who are preparing for their board exam because it compile the entire lesson into short and includes every important topics.
With the help of Haloalkanes and Haloarenes notes students can understand the chapter in a better way. Notes are prepared by very experience teachers in an organised way so students can rely on this notes for their exam preparation.
Class 12 Chemistry Haloalkanes and Haloarenes Handwritten Notes

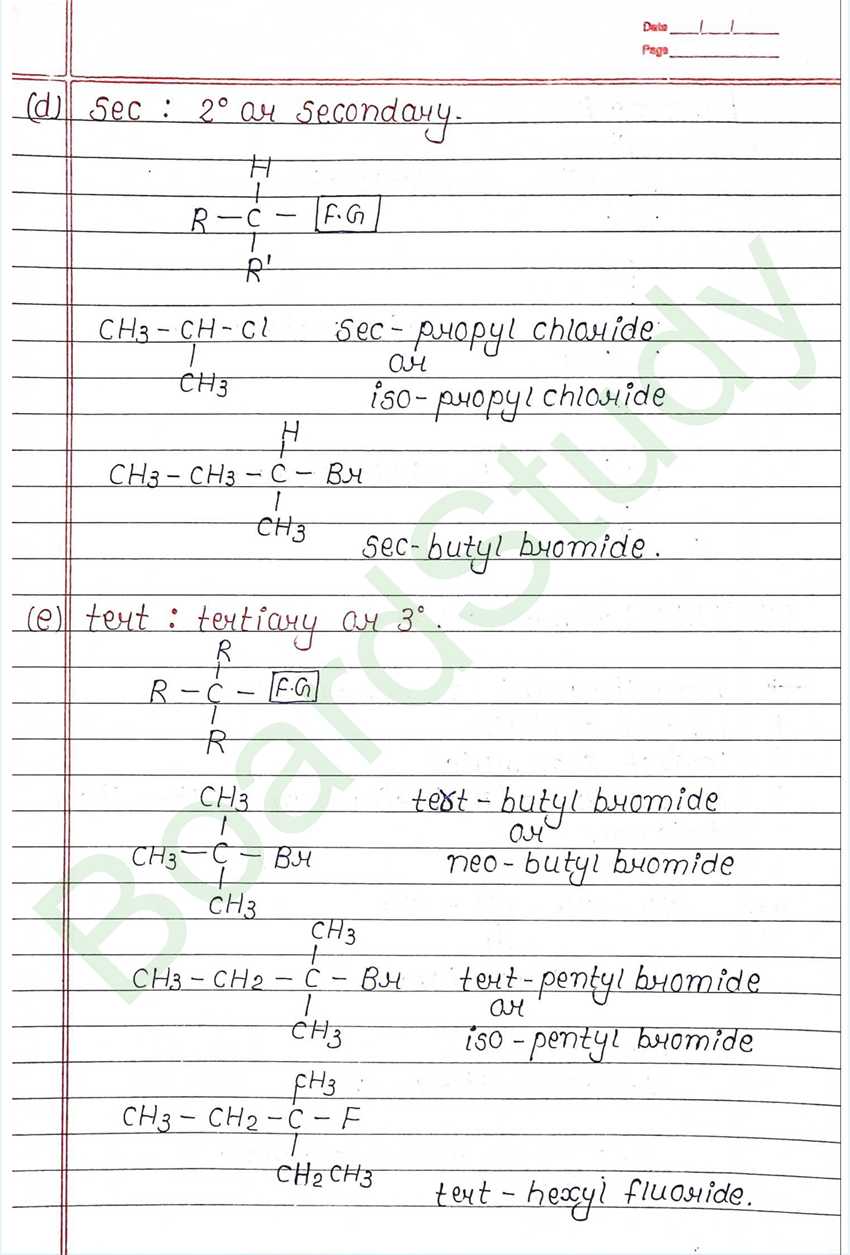
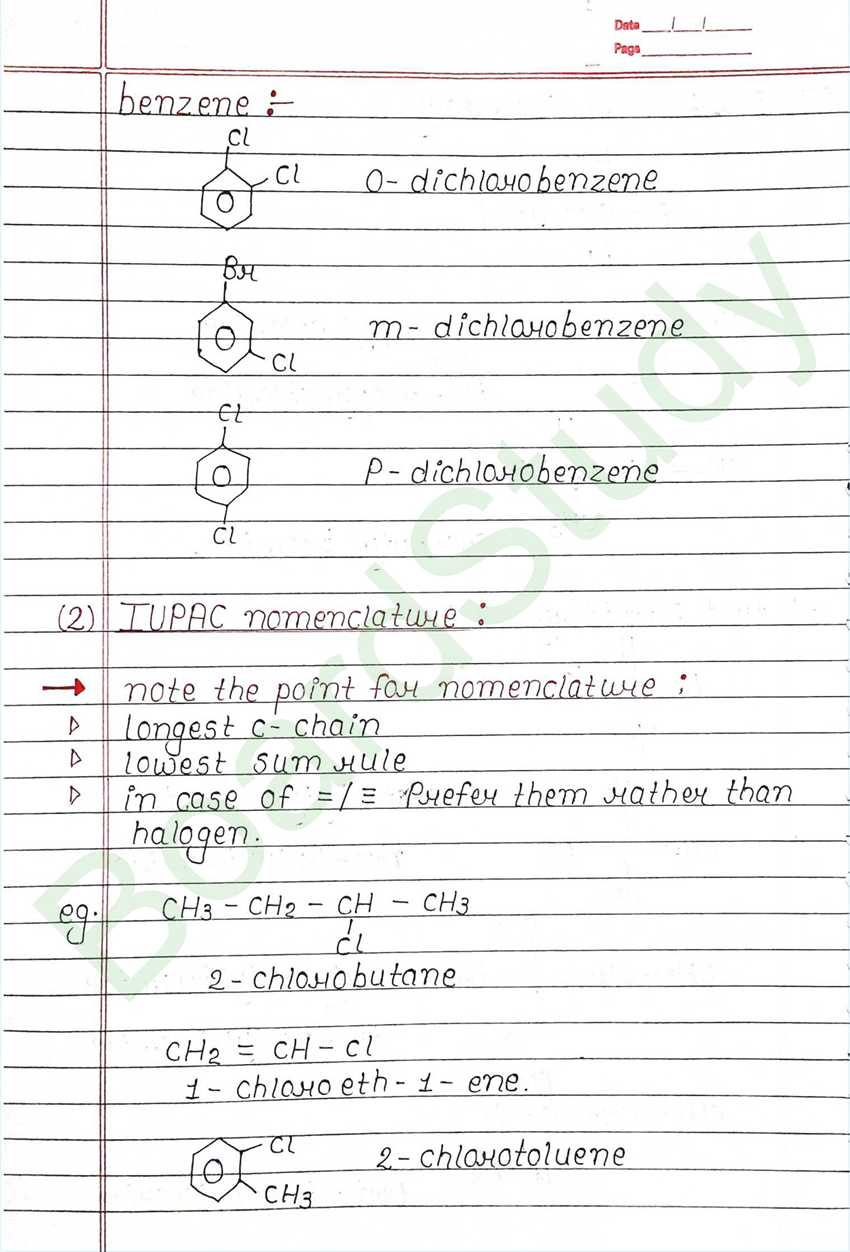
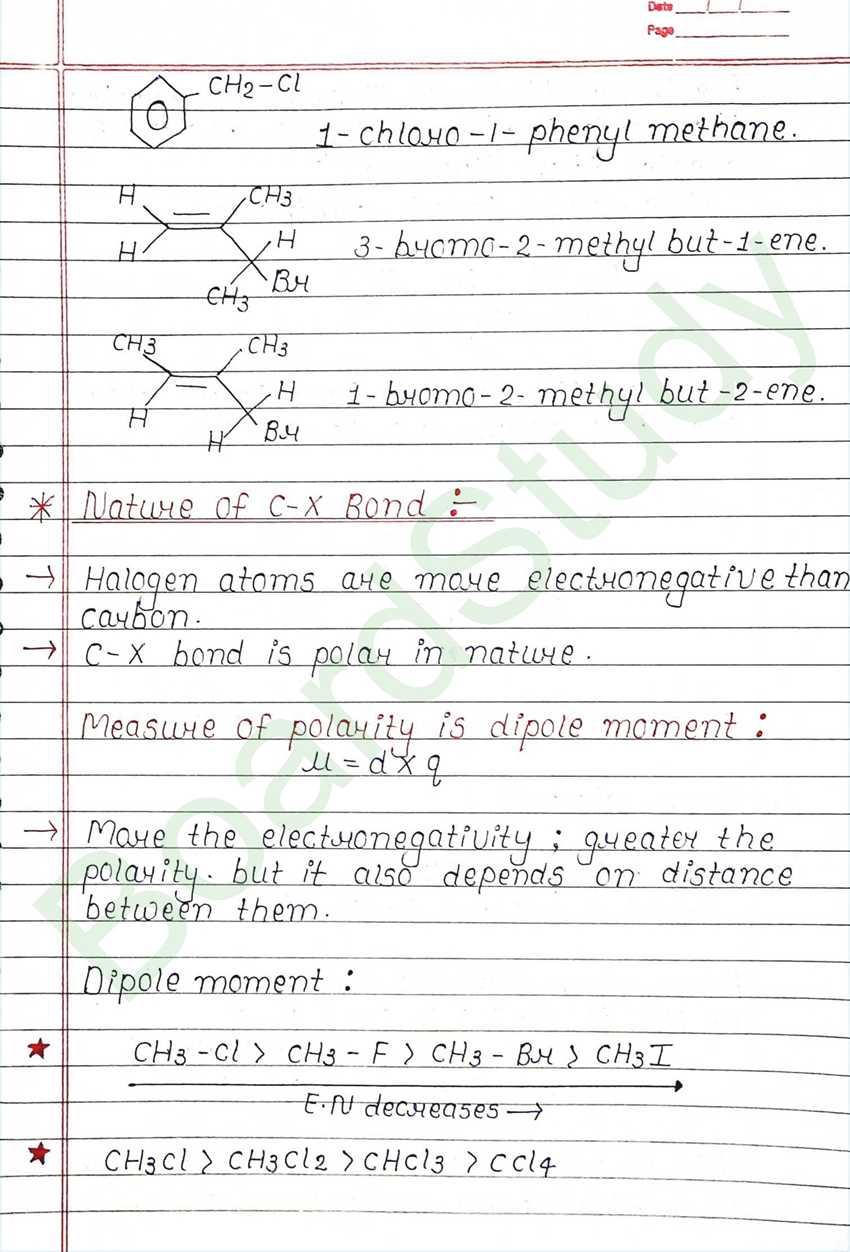
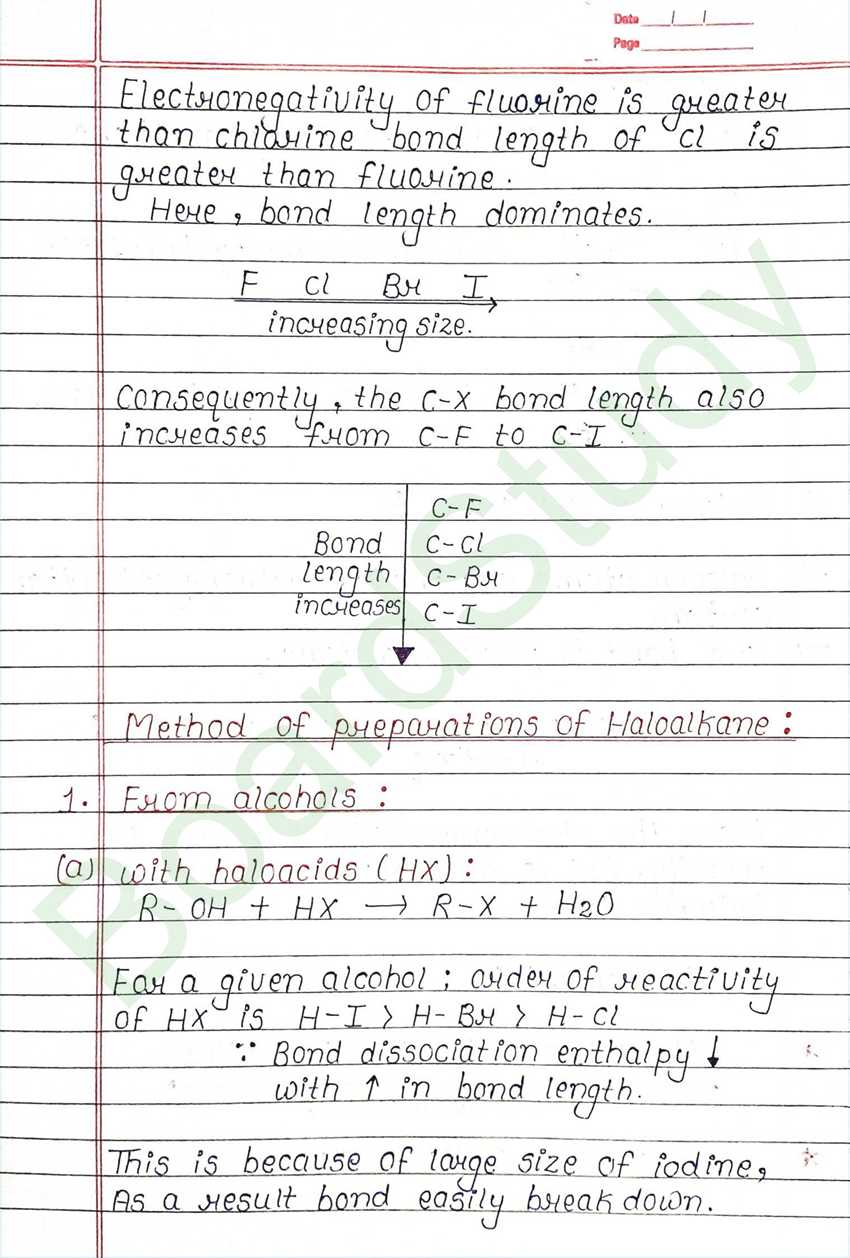
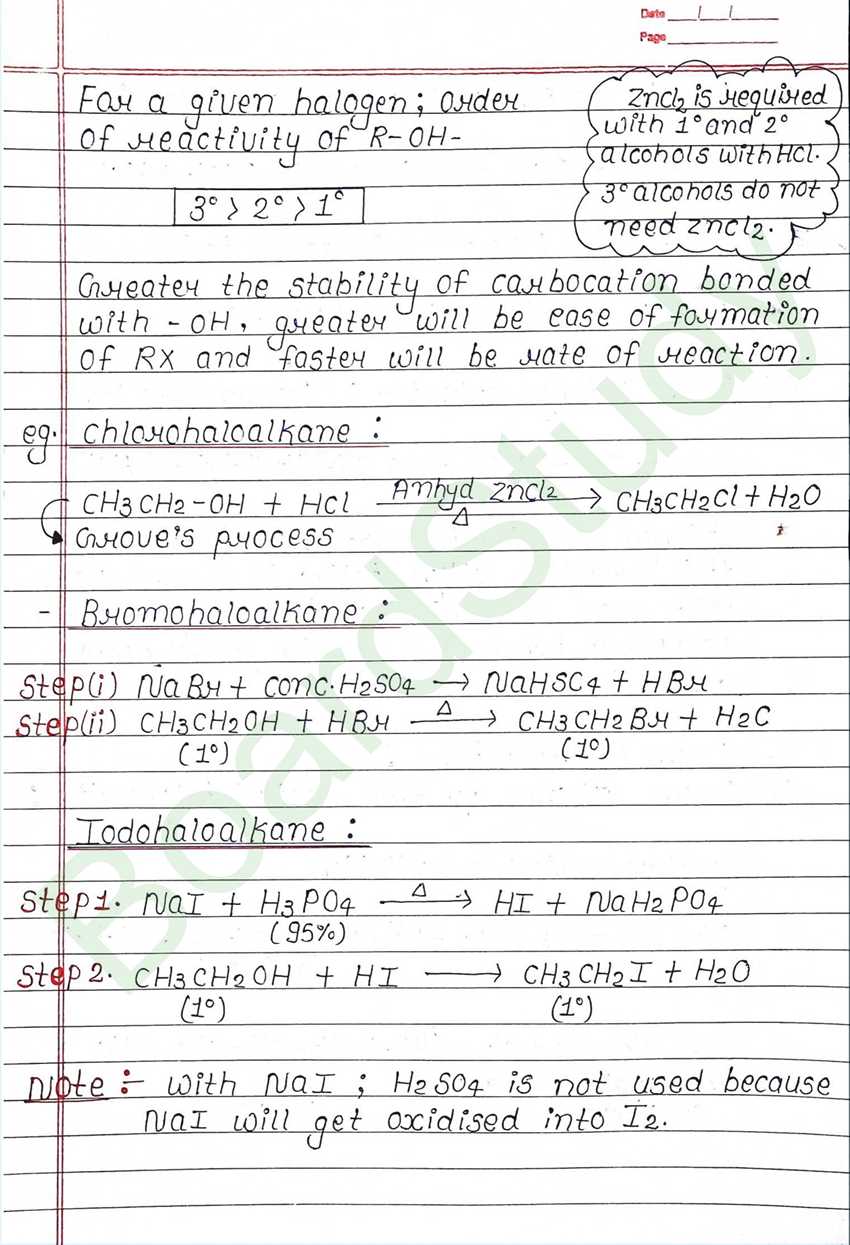


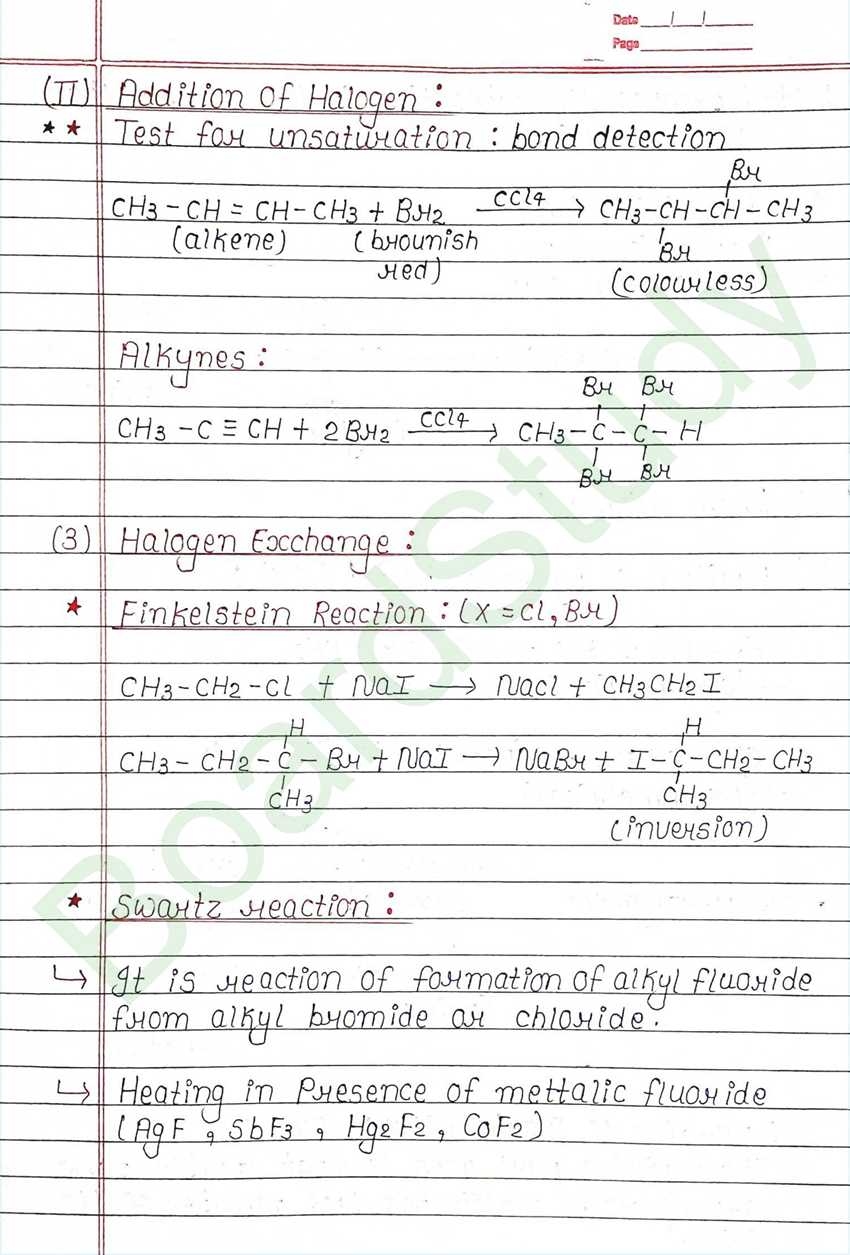
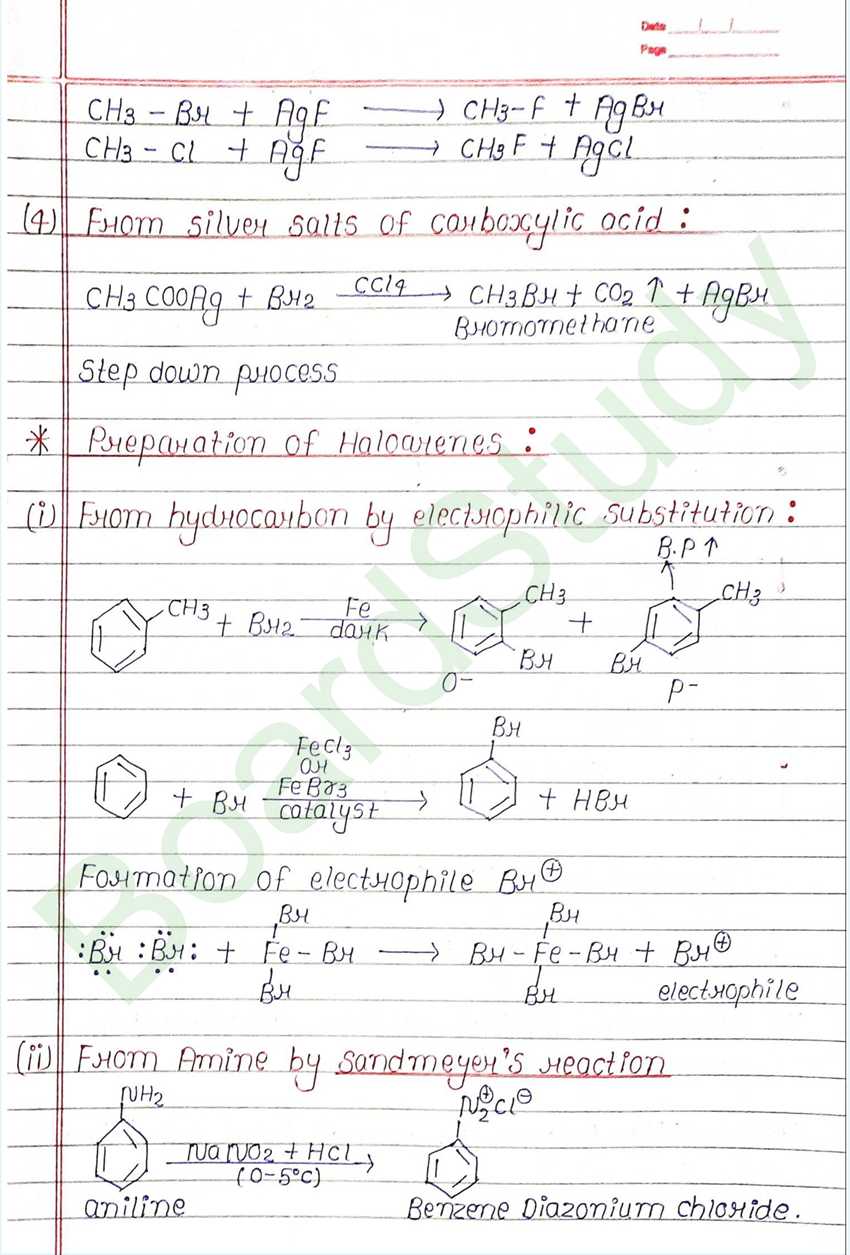
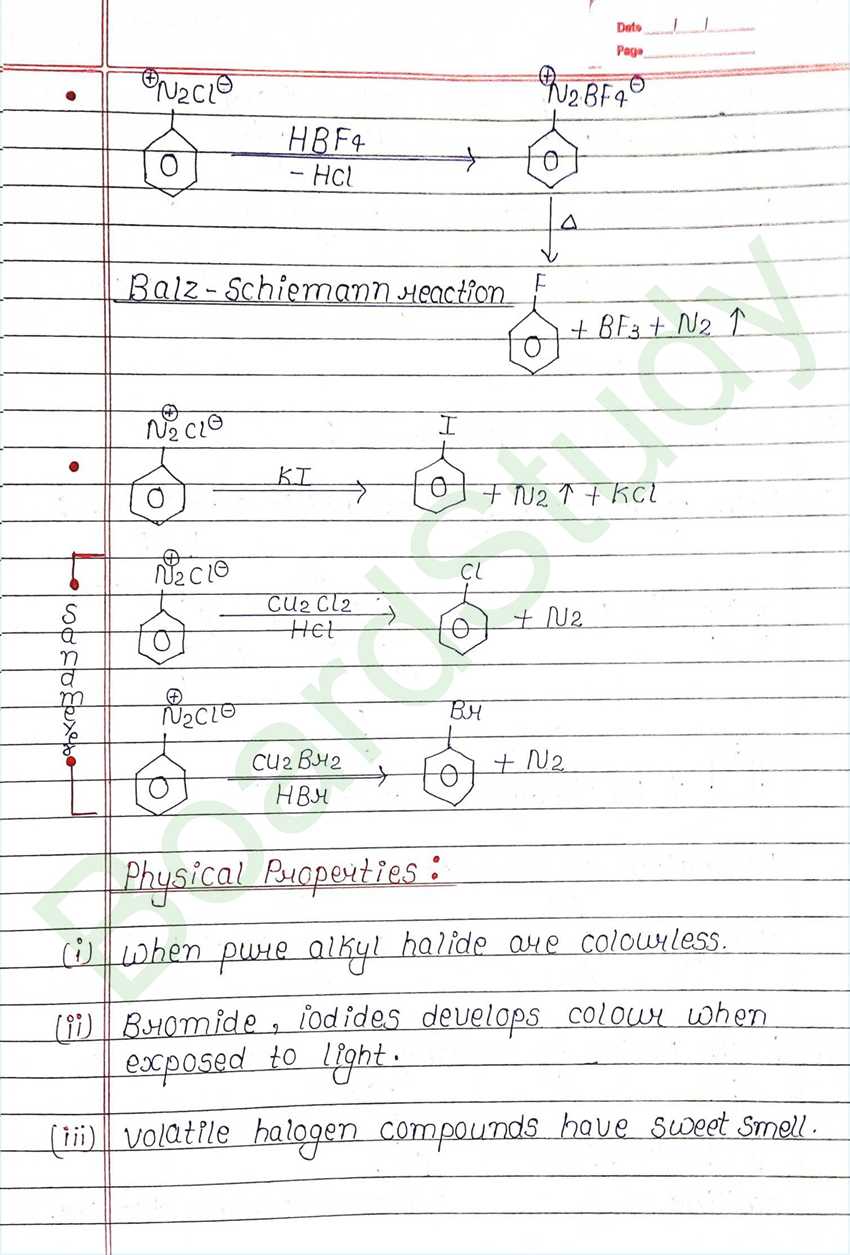













Next Chapter: Alcohols, Phenols, and Ethers
Previous Chapter: Coordination Compounds
Other Subjects:
Class 12 Biology Notes
Class 12 Physics Notes
Students can access this notes anytime on our website for free of cost. If you found notes helpful, you can also help your friends by sharing with them.
Key Points: Haloalkanes and Haloarenes Notes PDF
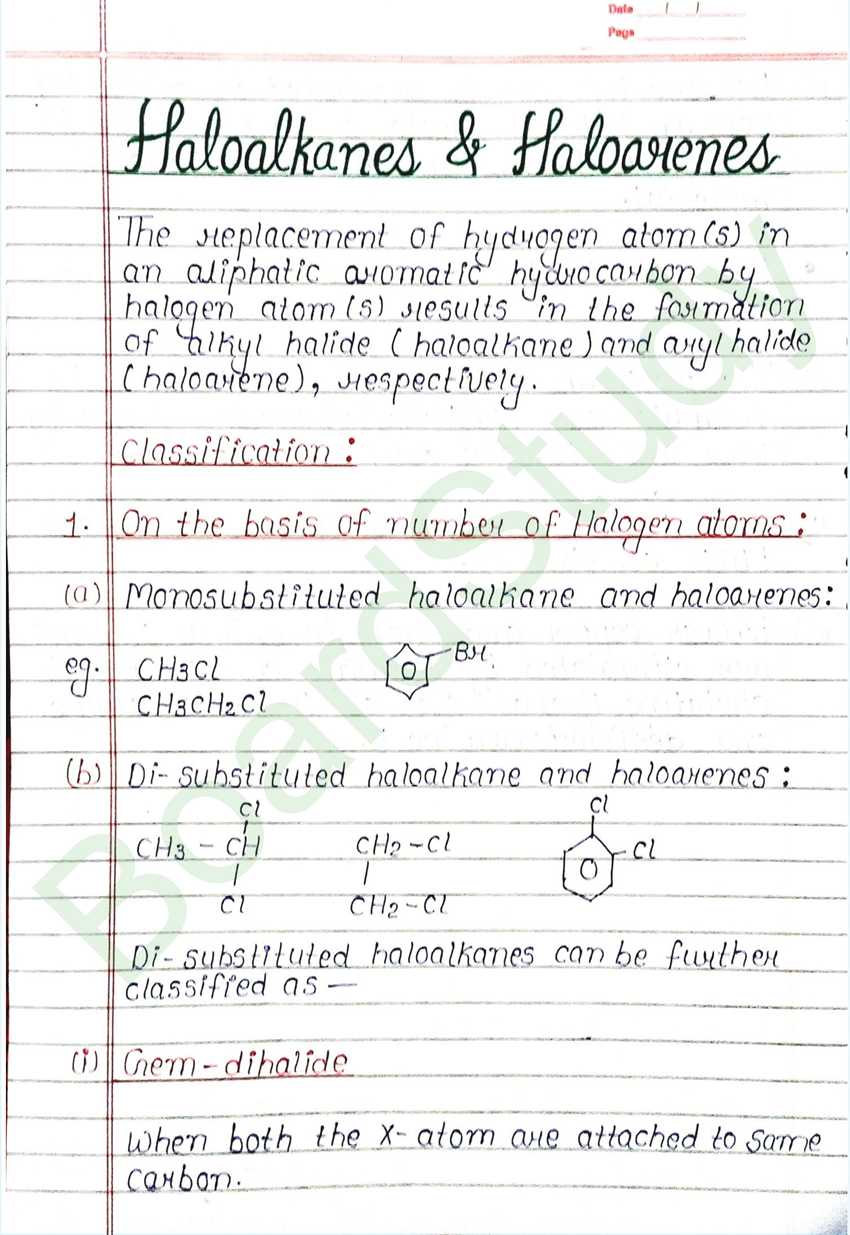
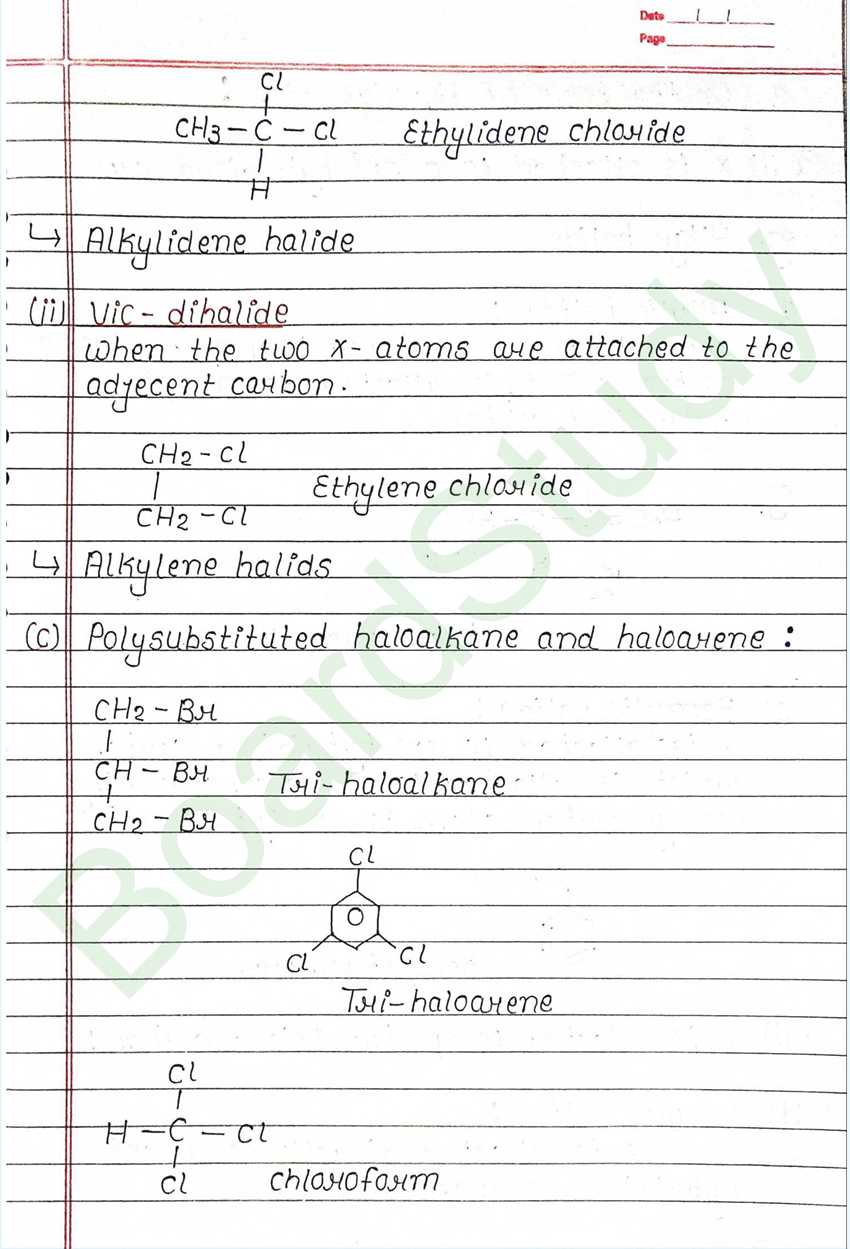
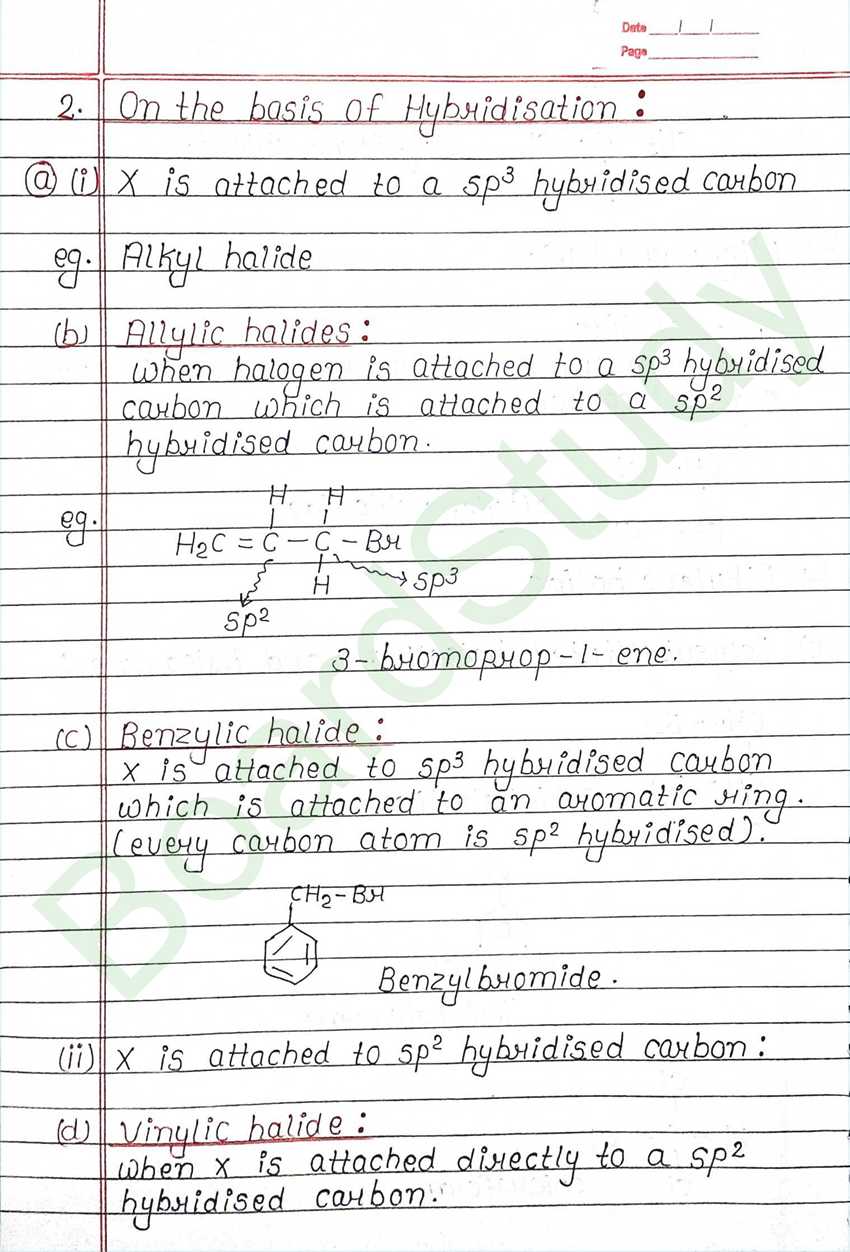
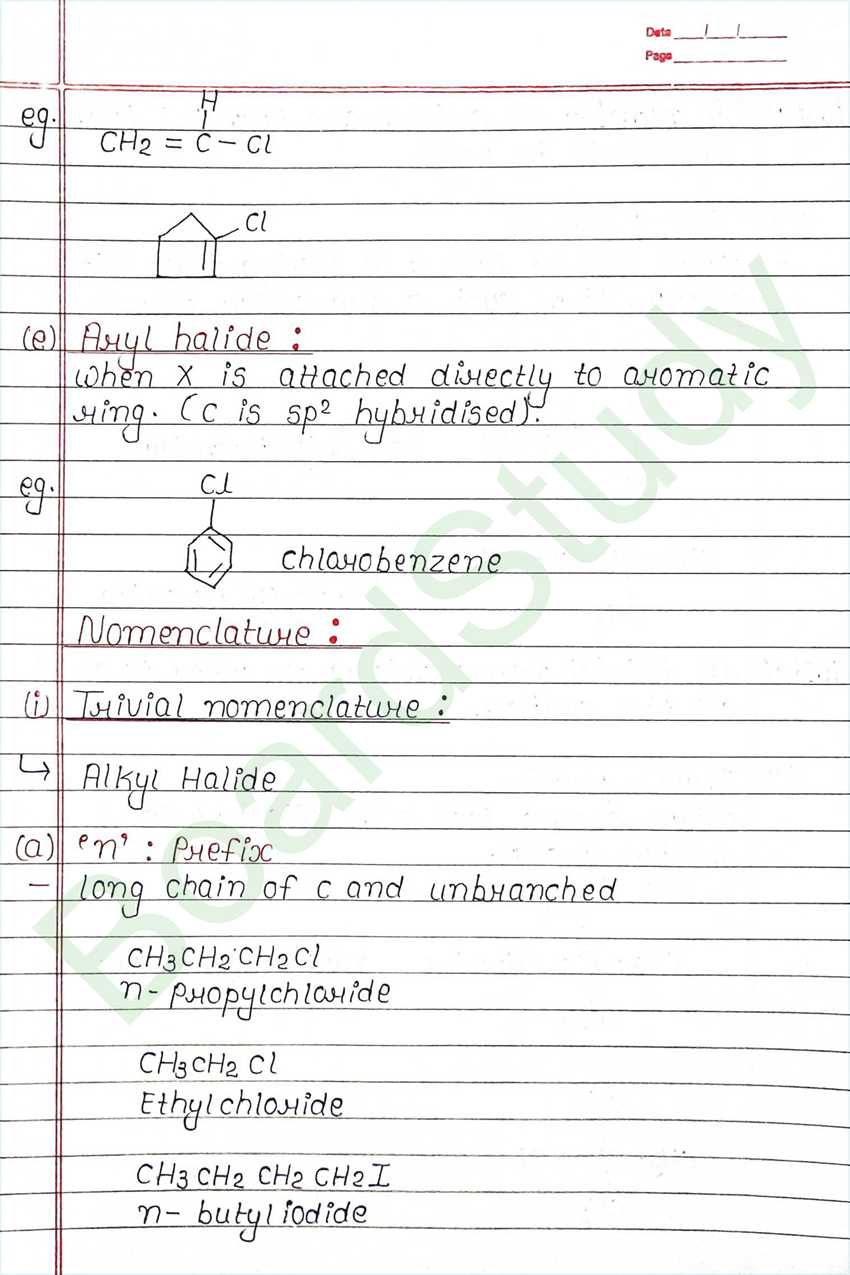
Haloalkanes & Haloarenes
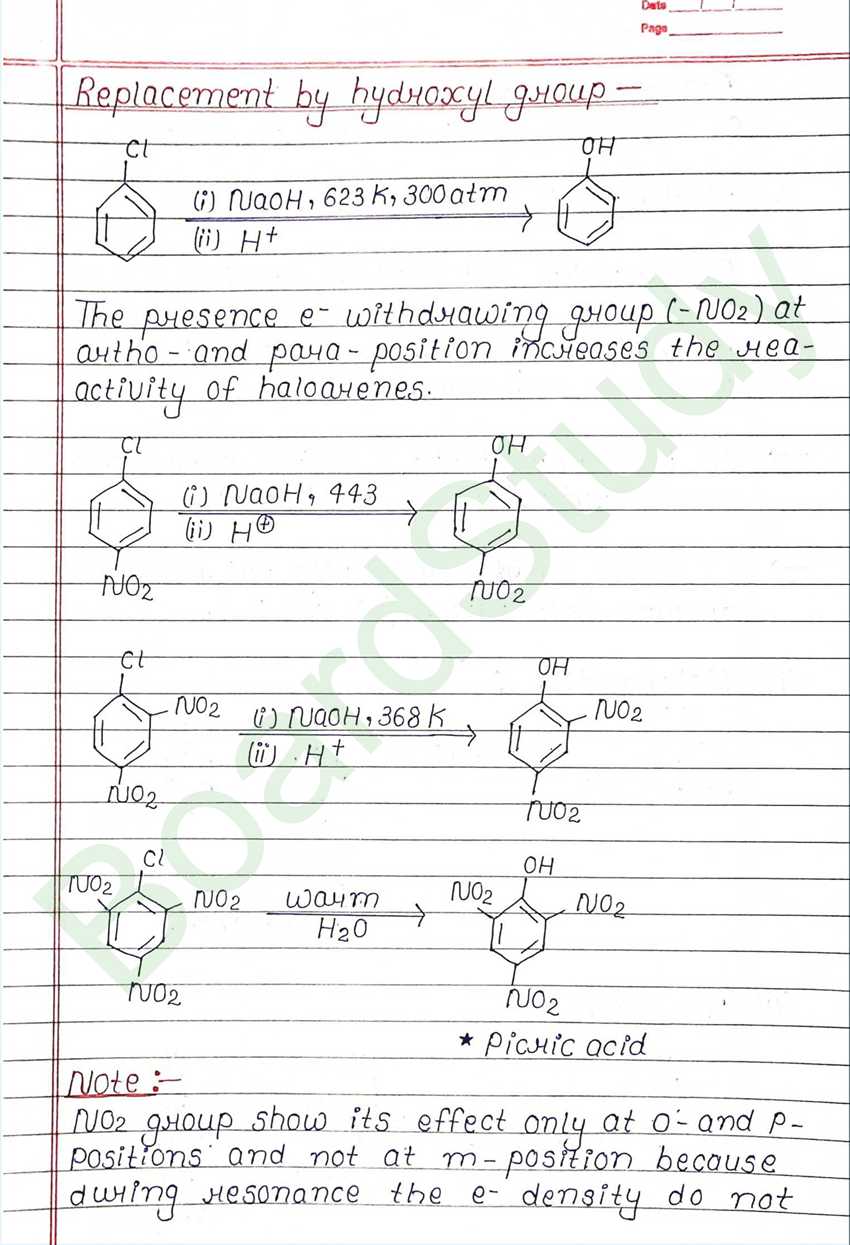
The replacement of hydrogen atom (s) in an aliphatic aromatic hydrocarbon by halogen atom (s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
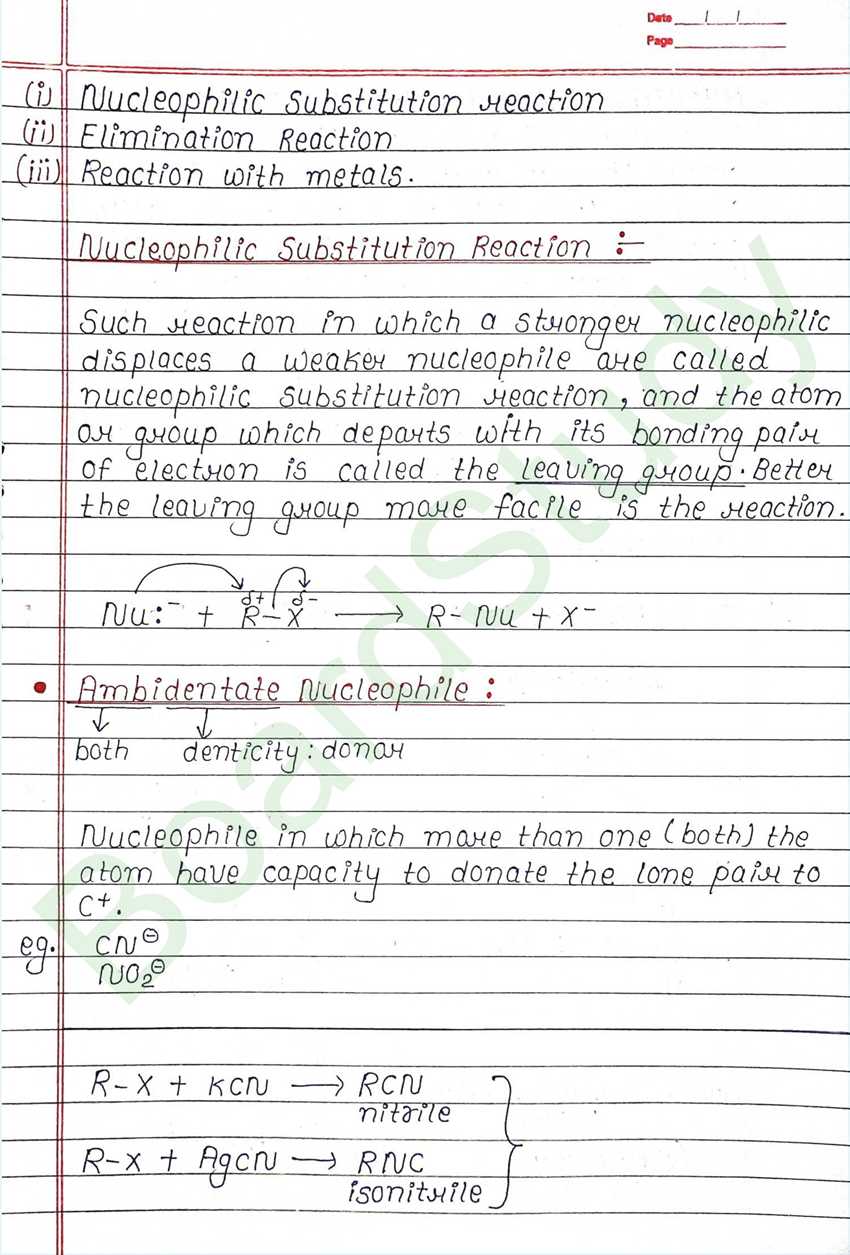
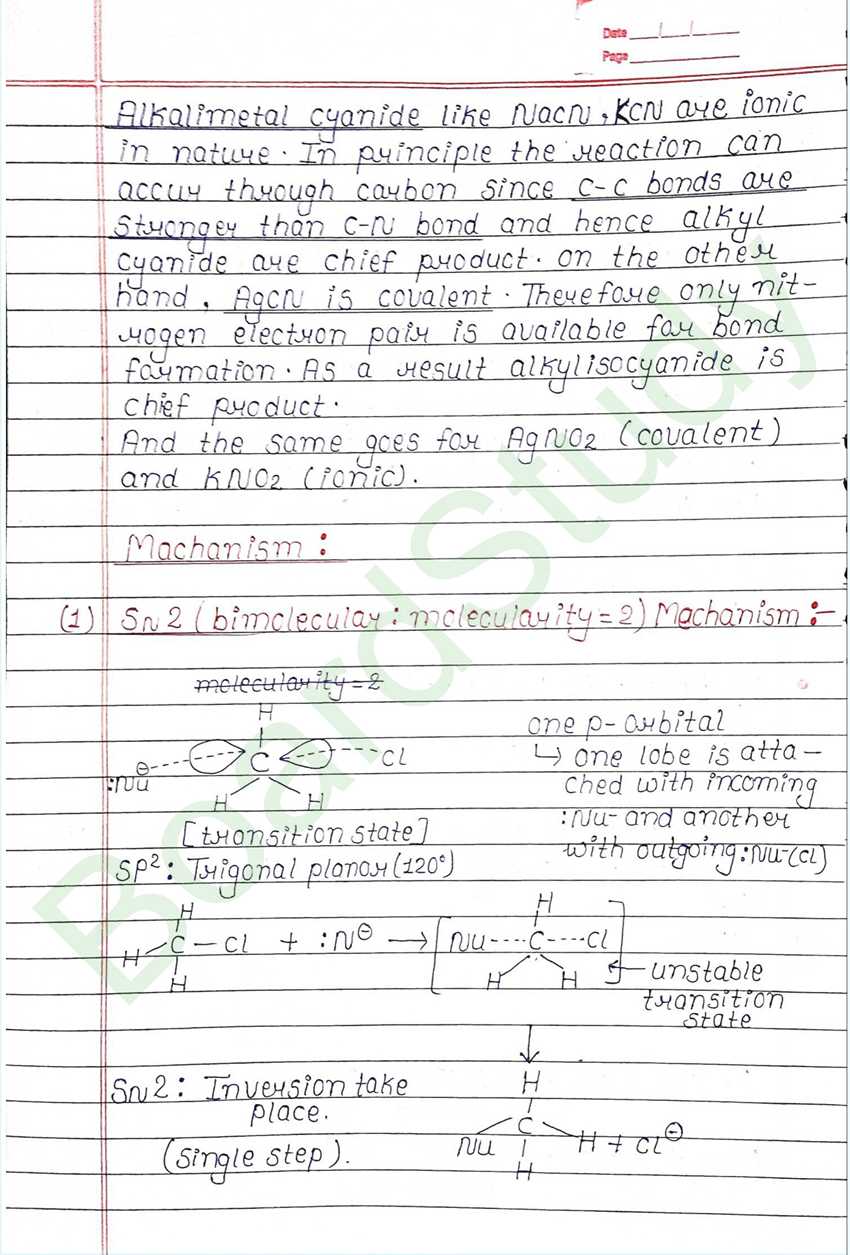
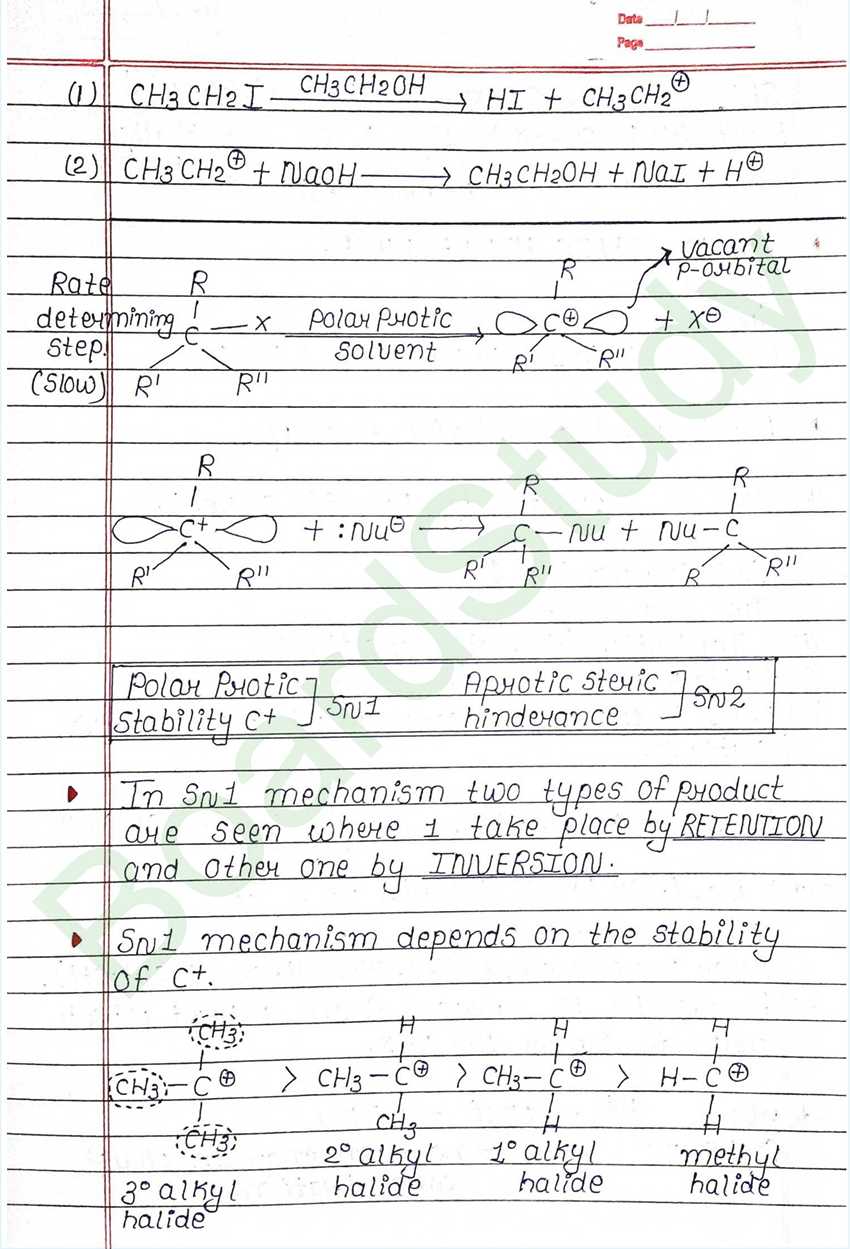
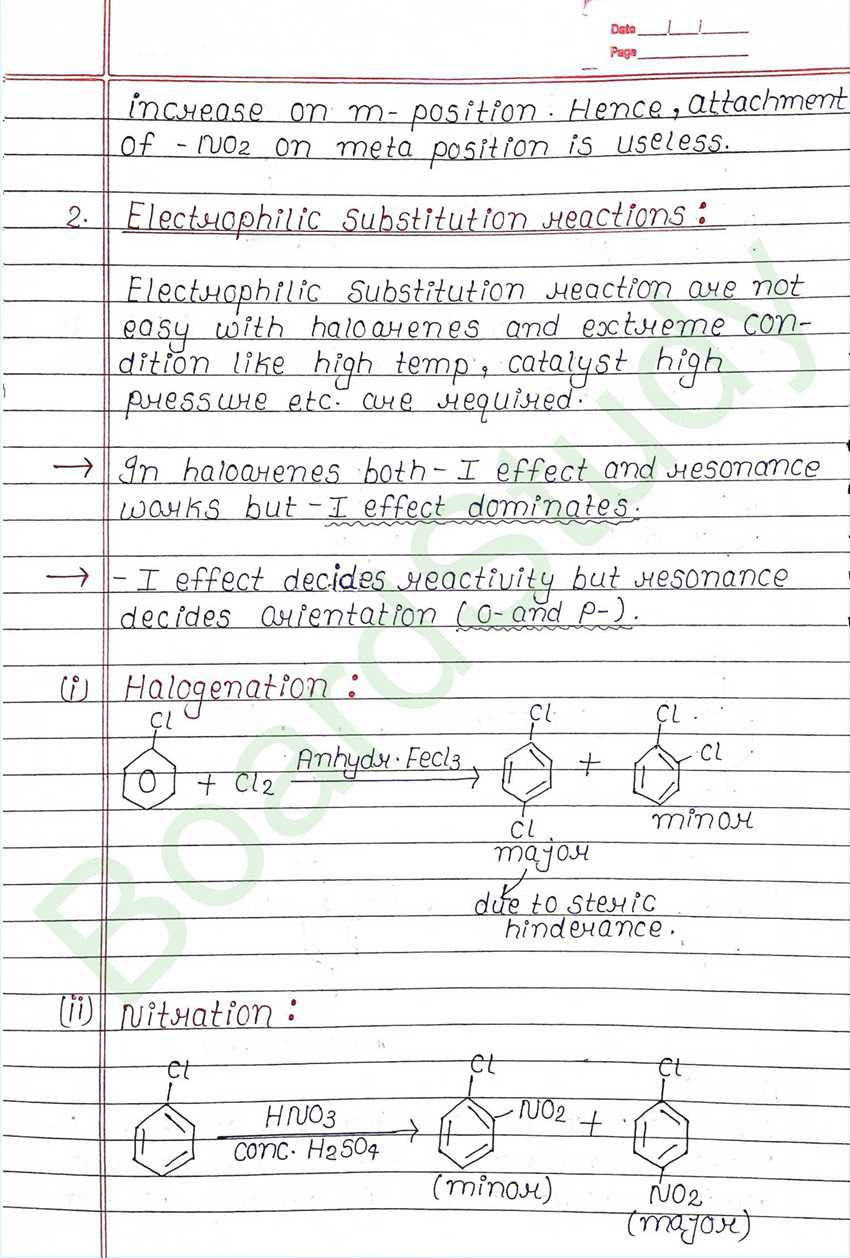
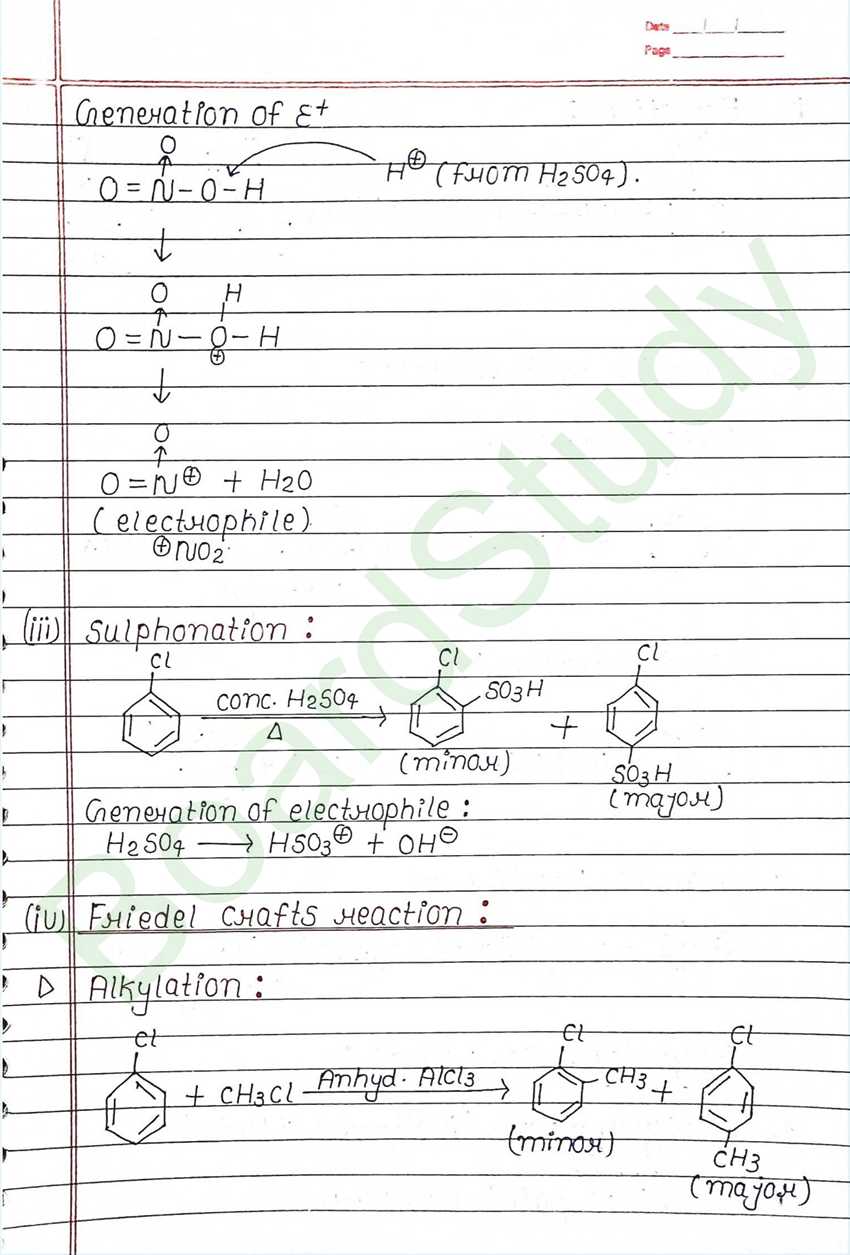
Nucleophilic Substitution Reaction :
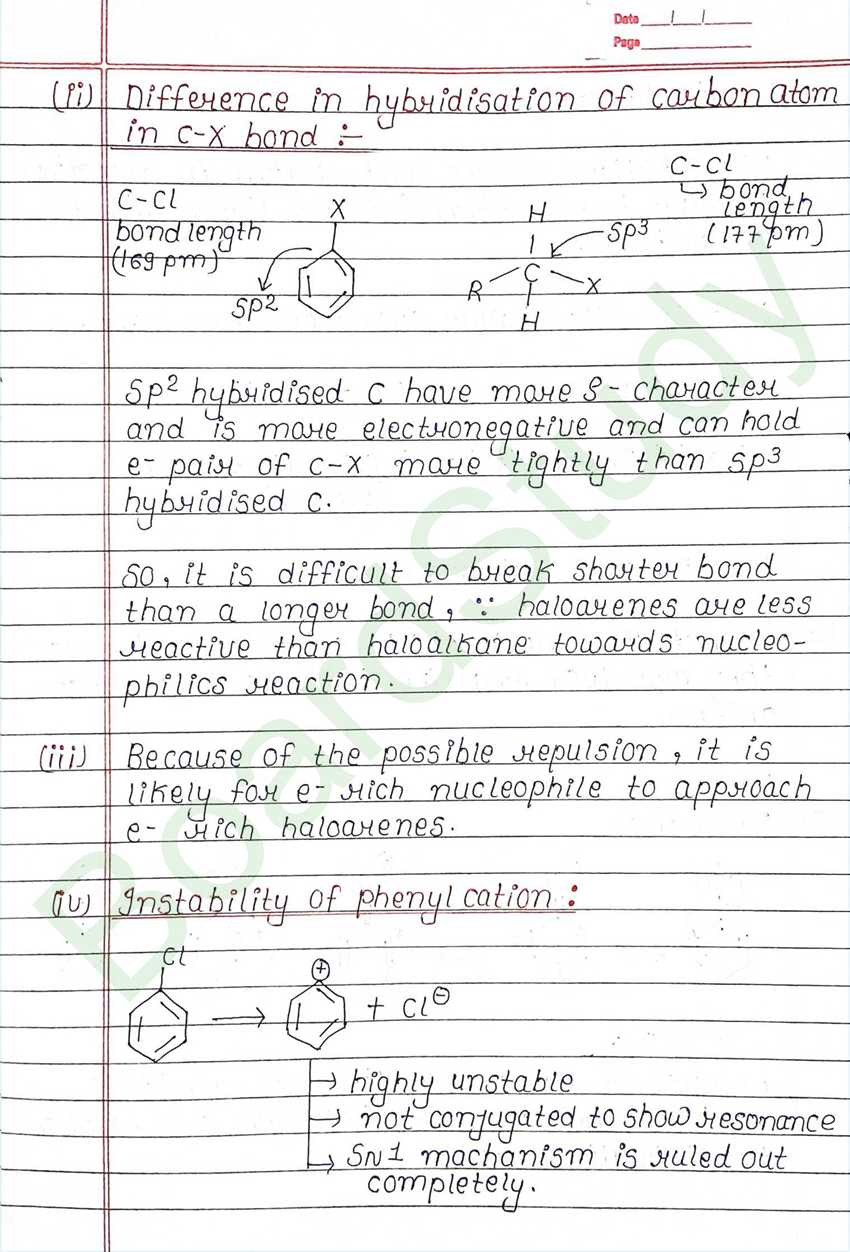
Such reaction in which a stronger nucleophilic displaces a weaker nucleophile are called nucleophilic Substitution reaction, and the atom or group which departs with its bonding pair of electron is called the leaving group. Better the leaving group mare facile is the reaction.
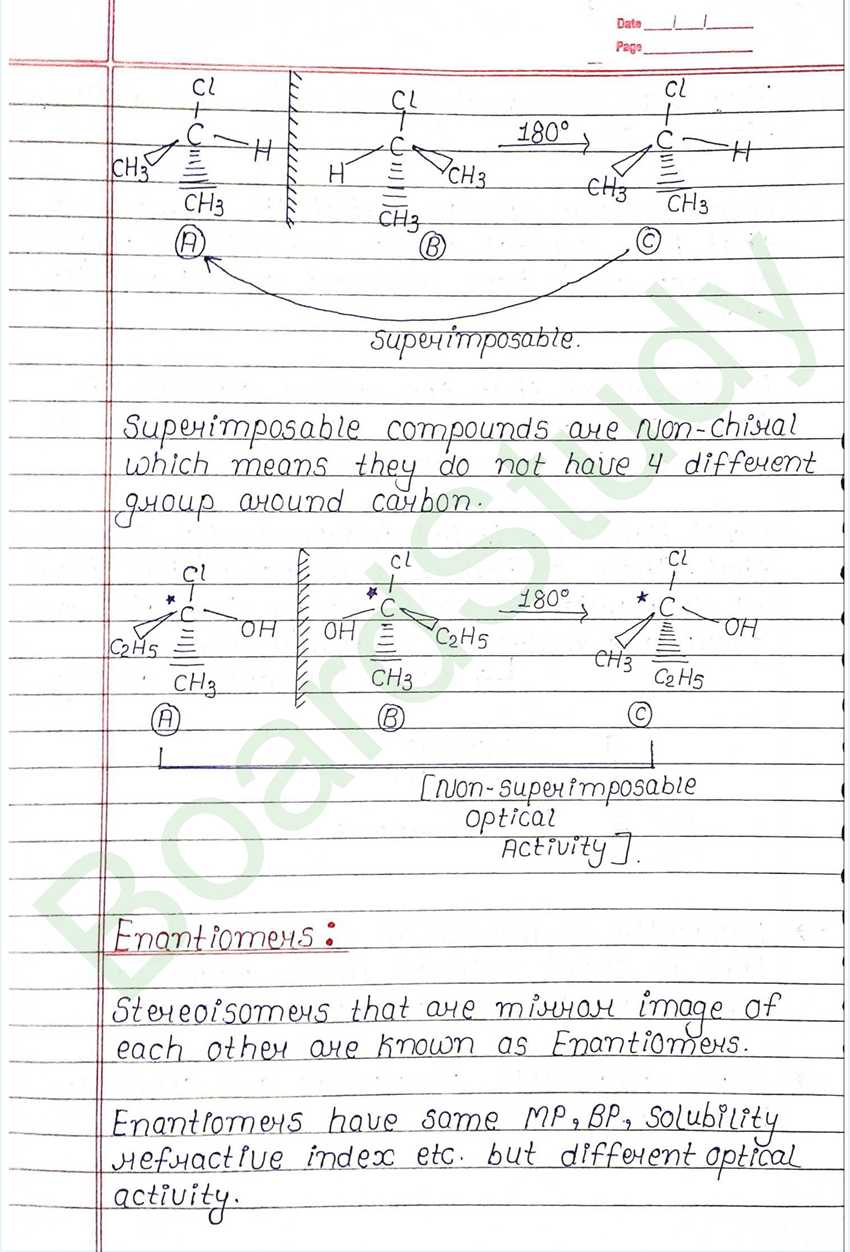
Optical Isomers : Compound that have same molecular formula but have different optical activity. i.e. one is dextro and other is laevo. This phenomenon is called Optical Isomerism.
Molecular asymmetry, chirality and enantiomers :
- Louis pasture observed that the compound that have crystal which are mirror images of each other have different optical activity also
- He suggested that the optical activity is because of different in 3D arrangement of atom in their molecule.
- Van’t Hoff and Le Bel independently suggested that. Those carbon in a molecule that have all four valancies satisfied by four different group are molecular asymmetric.
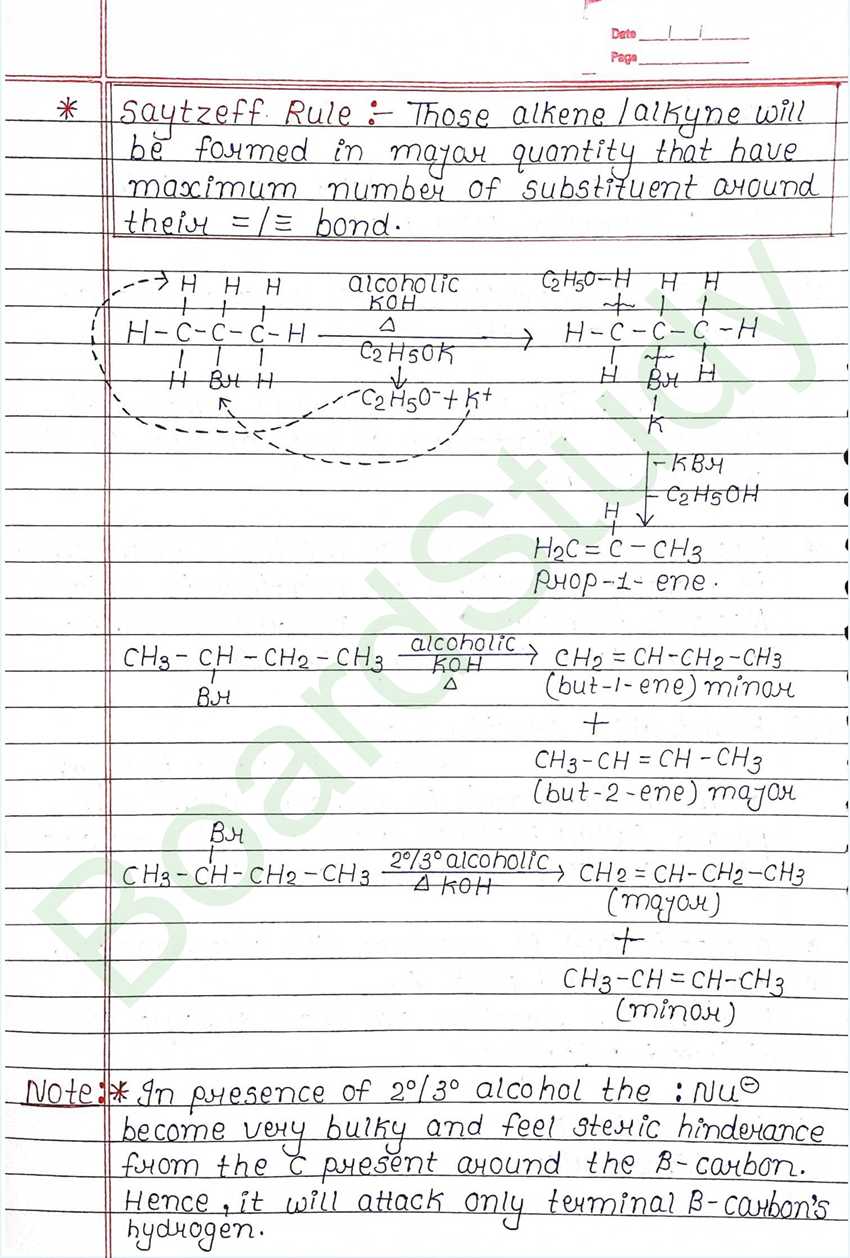
Those alkene /alkyne will be formed in mayor quantity that have maximum number of substituent around their = / = bond.
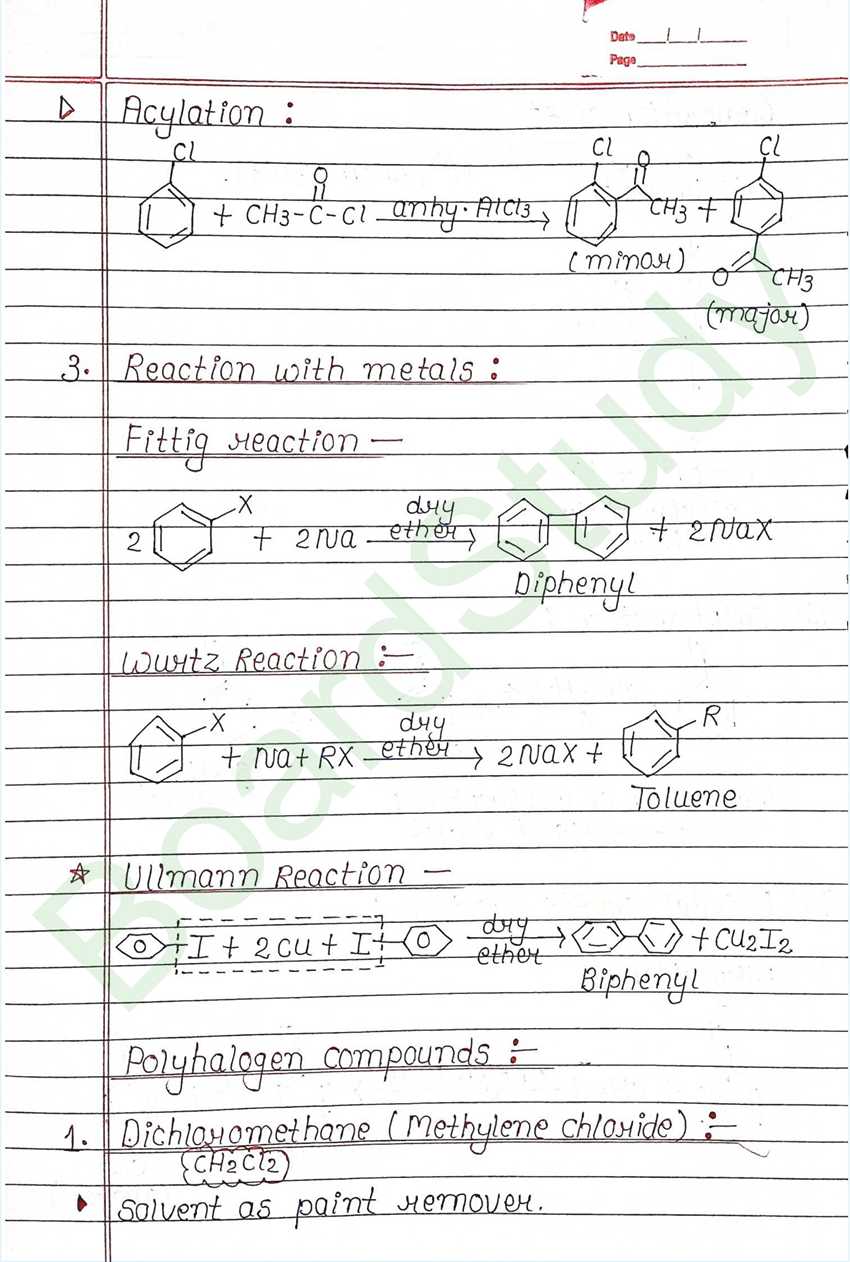
Reaction with metals:
Alkyl chloride, alkyl bromides and alkyl iodides react with Mg in presence of dry ether to form an organometallic compounds known as Grignard reagent.
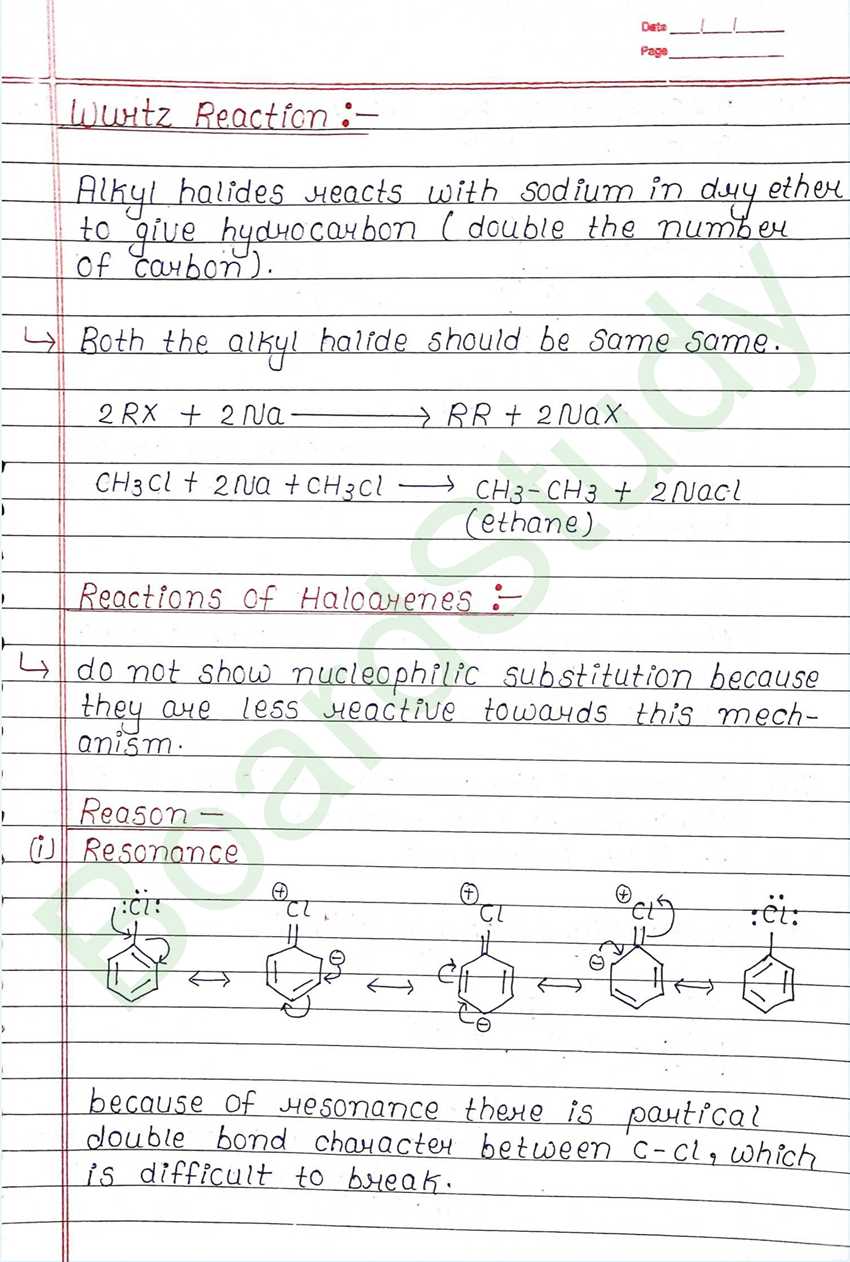
Wurtz Reaction:
Alkyl halides reacts with sodium in dry ether to give hydrocarbon (double the number of carbon). Both the alkyl halide should be same same.
Polyhalogen compounds :
Dichloromethane (Methylene chloride) : (CH2Cl2)
- Solvent as paint remover.
- Propellant in aresols
- Solvent in drug manufacturing
- Metal Cleaning and finishing Solvent.
- It harms C.N.S.
- Exposure to lower level in air can lead to impaired vision and hearing.
- High level – diziness, Nausea, tingling, numbness in finger and toes.
- Skin burning and mild Medness. Burn cornea.
Trichloromethane (chloform) :- (CHCL3)
- Solvents for fats, alkaloids iodine etc.
- General anesthetic in surgery (depresant-CNS) but replaced by ethers (less toxic).
- Used for production of FREON REFRIGERANT-22.
- Breathing 900 ppm cause fatigue, headache and diziness.
- Chronic CHCl3 exposure cause damage of the Liver / kidney and somes of skin.
Freons:
- The CFCs of methane and ethane are collectively known as Freons.
- Extremely stable, unreactive, non-toxic, non-corrosive and easly liquifiable gas.
- Freon-12 (CCl2 F2) – most common farm in industries.
- It is manufactured from ccl4 by swartz reaction.
- In atmosphere, remain unchanged in stratosphere and initiate Os layer damage by radical chain method.
Features of Notes
- Students can use Haloalkanes and Haloarenes notes for last minute revision.
- In the last few days of exam students feel very stress due to pressure of exam. Notes will be very helpful for managing the stress in the last days of exam.
- All notes are totally free of cost and students can access notes anytime on our for totally free of cost.
- Haloalkanes and Haloarenes Notes PDF are created very carefully so you can rely on this notes.
Summary
| Chapter | Haloalkanes and Haloarenes |
| Chapter Number | 6 |
| Subject | Chemistry |
| Class | 12 |
| Medium | English |
FAQ
What is Haloalkanes and Haloarenes ?
The replacement of hydrogen atom (s) in an aliphatic aromatic hydrocarbon by halogen atom (s) results in the formation of alkyl halide (haloalkane) and aryl halide (haloarene), respectively.
What is Wurtz Reaction ?
Alkyl halides reacts with sodium in dry ether to give hydrocarbon (double the number of carbon). Both the alkyl halide should be same same.
Are these notes sufficient for board exam?
Haloalkanes and Haloarenes handwritten notes are created by topper’s and expert teacher keeping board exam in mind so you can score maximum in board exam.
Are Haloalkanes and Haloarenes Handwritten notes according to NCERT latest syllabus?
Yes notes are created according to the NCERT latest syllabus.
How can i download Haloalkanes and Haloarenes Notes PDF?
For downloading Haloalkanes and Haloarenes Notes PDF click on Download PDF button.

nice
very cool